5 The “Memory” of a Cricket’s Immune System
Angelena Lara and Ben Spector
Abstract:
Crickets have complex immune systems which are not yet fully understood. The adaptive qualities of the cricket immune system are known as immune priming, and it helps prepare against an immune challenge. Immune priming increases the strength of immune response after exposure to a disease or pathogen. This can also lead to specification, which means an individual has developed the tools needed to fight of a specific infection. The degree to which immune priming specifies is known as specificity. We wanted to better understand the extent of specificity in our cricket system, and further develop knowledge of adaptive immunology in insects. In this experiment, we put crickets into three treatment groups (a lipopolysaccharide (LPS) injection, a sham injection, and a non-injection), and three days later, inserted them with a nylon thread to determine their ability to melanize. The LPS group served as our challenge group in order to determine if an immune challenge would result in higher encapsulation. The experiment found that encapsulation rates across the treatment groups were similar, meaning that the LPS injection had no effect on the rate of encapsulation. It also found that crickets who had been injected with LPS or the sham injection had higher mortality rates than the non-injection group, meaning that the process of injection caused a high mortality rate.
Background:

What is the immune system of a cricket? How does it work?
The immune system of a cricket can be split into two pathways, cellular and humoral. The cellular pathway is based around hemocytes, which can phagocytize or “eat” small pathogens and eliminate them. If the pathogen is too big, then the hemocytes can bind together and form a layer of coating to isolate the pathogen from the rest of the body. This process is known as encapsulation, and includes the other part of the immune system, the humoral. In the humoral pathway antimicrobial peptides break down bacterial cell walls. When the two pathways work together we call it nodulation. This occurs when the hemocytes surround a portion of bacteria and melanize around it to capture the bacteria, then the humoral pathway takes over to help eliminate the threat. The melanization that takes place can be used to measure how effective an immune response is.
How is a cricket’s immune system different from other animals?
Both crickets and animals have humoral and cellular pathways to help fight disease, but vertebrates have a special third type of immune system called “adaptive immunity”. This adaptive immunity helps the vertebrate’s body recognize and fight off specific pathogens, no matter what they are. Even though a cricket’s body has an adaptive ability, immune priming, this is different from a vertebrates because they have to come in contact with the specific pathogen first.
What is immune priming?
Immune priming is a cricket’s version of adaptive immunity. It lets a cricket fight an infection better after it’s first encounter with that specific infection.
What is specificity?
Specificity is a part of immune priming. It is how well immune priming can separate different pathogens and see how related they are. If immune priming is highly specific then it would only be able to fight off one specific pathogen really well. But if it came across a pathogen that was just a little bit different it would have to start fighting it from square one. If immune priming isn’t specific at all, then every time a cricket found a new pathogen and fought it off it would get slightly better at fighting all pathogens. Think of it like a video game, the more times a cricket fights an infection the more it ‘levels up’ and the better it is at fighting a disease.
How are you studying specificity?
Since we’re trying to figure out how specific a cricket’s immune priming is, we introduced the crickets to two different challenges, one after the other. Our first challenge had three
different treatments; lipopolysaccharide (LPS), a sham injection made of Grace’s insect medium, or no injection at all. LPS is a toxin found on the surface of Gram-negative bacteria, such as Serratia marcescens, the source of our LPS. Injection with this effectively simulates an attack by S marcescens. The injection is sort of like a vaccine, because it introduces the cricket body to a pathogen at a low enough dose that the cricket should be able to fight it off. The sham injection made of Grace’s insect medium acts like cricket saline, so that we could see if injection had an effect on encapsulation. The non-injection group didn’t have any injection so we could have a control group. After a few days we implanted the crickets with a nylon thread for a few hours, when we removed them we were able to see how much melanization had occurred. This type of challenge simulates a parasitic attack, like that of a nematomorph worm parasite that A. domesticus may encounter in nature. The melanization shows how much encapsulation had occurred, and by measuring that we could tell which of the three treatment groups was best at fighting off the infection.
Significance:
Since immune priming is a factor of the insect immune system which is similar to the adaptive immunity in vertebrates, we hope to gain a better understanding of it. The ability of immune priming to strengthen immune response varies across species. Evidence has been found of priming specific to an initial pathogen like a certain bacteria type, but also of priming that is broad enough to protect against a completely different challenge such as a virus, fungus or parasite. Our research gave us a better idea of how specific immune priming is in our model species Acheta domesticus. Our results uncovered more about this important part of innate immunity and allows us to compare it to the vertebrate immune system.
Research Goal:
We hoped to determine if immune priming by Lipopolysaccharide (LPS) molecules from a bacteria provided immune priming in Acheta domesticus crickets that was broad enough to protect against different types of immune challenges, such as a parasite.
Methods:

- This experiment started by weighing and recording the sex of 30 adult crickets.
- We then injected them with 10 μL of their treatment.
- LPS solution at LD-50
- Grace’s insect medium
- No injection
 The crickets were put in a small individual deli container with an egg carton, Fluker’s feed cubes, and a small petri dish. They stayed there for four days, but were checked on every day to replace food and catalog deaths.
The crickets were put in a small individual deli container with an egg carton, Fluker’s feed cubes, and a small petri dish. They stayed there for four days, but were checked on every day to replace food and catalog deaths. - After day 4 the crickets were ready for part two of the experiment; the parasite phase. For this part of the experiment we roughed a strip of nylon thread and cut it into 1 cm strips with a little knot on the end.
- We froze the crickets for four minutes, then wiped them down with ethanol and removed one of their legs. We removed a leg because the crickets would try to remove the nylon themselves, stopping all melanization and data collection
- We made a small hole in the cricket’s abdomen using a sterilized straight pin, then put the nylon thread inside.
- After four hours the nylon thread pieces were taken out, labeled and dated, then moved to a small container to be analyzed later.
- A few days later we took pictures of the threads and analyzed them in a program called ImageJ, which let us see how dark the threads were.
Results & Interpretation:
- We found no significant difference in abili
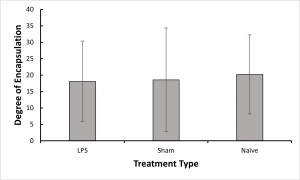 ty to encapsulate the nylon thread between our treatment groups, which suggests that if immune priming was created by our LPS challenge, it was not broad enough to strengthen response against a parasite.
ty to encapsulate the nylon thread between our treatment groups, which suggests that if immune priming was created by our LPS challenge, it was not broad enough to strengthen response against a parasite.
- There was also no significant difference in
 encapsulation ability between male and female crickets
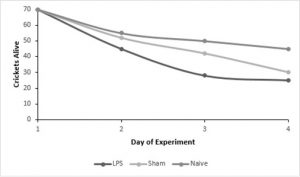
encapsulation ability between male and female crickets - We experienced a large amount of mortality, reducing our initial sample size of 210 crickets to 84 crickets by the end of our experiment.
- Mortality was significantly higher in crickets that received either injection (LPS or sham) but there was no significant difference between those two treatments, suggesting that injection itself increases mortality.
- Male and female crickets did not differ in mortality.
- There was no correlation between weight and encapsulation ability.


………………………………………
.wor………………………………………………………………………………………………………………………………………….
Future research & applications:
It is possible that our immune challenge, the LD50 dose of LPS, was too strong to prime against future immune challenges, and that crickets expended too much energy fighting the challenge to be able to devote energy to strengthening immune responses. A smaller immune challenge may have delivered different results, and would be worth studying. Reducing the strength of the immune challenge may also have the effect of reducing mortality, which was very high in our experiment, and a larger sample size would make our results more convincing. It is also possible to induce priming through other methods of exposing crickets to a challenge, such as through diet, which could better simulate natural exposure to pathogens. Although broad immune priming did not occur to strengthen encapsulation in our experiment, other factors of immune response, such as lysozyme activity or number of hemocytes could be measured to look for any modification of those responses.
Further reading:
https://www.frontiersin.org/articles/10.3389/fimmu.2017.00539/full#:~:text=their%20immune%20response.-,A%20Potential%20Mechanism%20for%20Acquired%20Immunity%20in%20Insects,the%20process%20of%20somatic%20recombination.&text=This%20observation%20suggests%20at%20least,long%2Dlasting%20protection%20in%20insects.
