7 To Reproduce or Not to Reproduce?
The Role of Food Availability and Immune System Activation in Determining Reproductive Investment
Mary Huynh; Camille Pushman; Matthew Ross; and Mikayla Stephens

Abstract
Because all-you-can-eat buffets are not found in nature, organisms have a limited supply of energy from food. Organisms must balance the amount of energy that they spend on different activities, such as their immune and reproductive systems. We wanted to test the effects of food availability and the strength of an immune challenge on reproductive effort. If an organism does have access to a buffet, does it have enough energy to fight off an infection and reproduce at the same time? Also, does fighting a severe infection pull more energy than fighting a minor infection? We chose to study male crickets (Acheta domesticus) as our model system. We injected the crickets with various doses of a molecule called LPS that activates their immune system. Then, we starved some crickets while giving others an all-you-can-eat buffet of cricket food. After that, we extracted chia seed-sized sacs of sperm called spermatophores from the crickets. We looked at the sperm under a microscope to see how many were dead or alive. We noticed that sperm viability decreased when crickets fought a strong “infection” of LPS. Surprisingly, having access to an all-you-can-eat buffet also decreased sperm viability. We also observed that crickets who had their immune systems activated by LPS were more likely to die and less likely to produce a spermatophore. Our findings are limited because we only obtained a small sample of spermatophores. Further experiments should test these findings. This research could help us to save threatened insect species and eradicate pests.
Background
As a human, you eat consistently to ensure you have the energy to carry out your normal functions like digestion, communication, sexual reproduction, and your immune system. Because we have grocery stores and do not hunt and gather for our food, not having enough energy to carry out our normal processes isn’t something most people worry about. Animals, however, still have to expend energy just to find their food in order to get energy. Because animals have limited amounts of energy, an issue arises when one function requires extra energy. For example, if an animal is sick and struggling with the energetic cost of an immune response, it may have less energy to put towards reproduction or searching for food. You can imagine, also, that if the animal had no food available, that they would have much less energy and these normal tasks would become significantly harder. These energetic cost imbalances are known as trade-offs.
When trade-offs occur, you often see an animal allocating less energy towards one function in order to take care of another. When you are sick and barely have enough energy to eat, animals feel the same when undergoing an immune challenge. For example, when an animal puts extra energy towards its immune response to keep itself alive, it may invest less energy into reproduction. You could assume that having less food around to eat for energy makes the animal’s body work much harder to maintain its normal processes. For some animals, if its body is under too much stress and is at risk of dying (and never getting the chance to reproduce again), it will focus its energy on reproduction instead of on staying alive. Queen honey bees produce more eggs as they get older and spend more energy on reproducing, because the older they are the greater chance there is that they might not be able to reproduce in the future (you can learn more about this here). This is known as a terminal reproductive investment, or TRI.
TRI’s can happen for lots of reasons- aging, not having enough food, or facing an immune challenge that makes them very ill. Some animals invest in reproduction by doing more mating behaviors or producing more sperm or eggs. These factors can show any fluctuations in reproductive investment, so when an animal invests more, like during a TRI, scientists know.
We wanted to know how food availability and strength of immune challenges affect the quality of reproduction in an animal. So, if they are exposed to more illness, will they invest less energy into reproduction because of tradeoffs? Or will they invest more, and go through a TRI? And if they have food, does that help them battle the illness better? Could having no food be too much stress and trigger a TRI?
In our experiment, we measured the amount of energy invested in reproduction by analyzing sperm. Our research subjects were male Acheta domesticus (A. domesticus) crickets, which produce tiny little chia-sized sacs of sperm called spermatophore. To trigger their immune system response, we injected them with a molecule called LPS. This molecule can activate their immune system without actually getting them sick, similar to how someone with allergies may feel sick but not actually be sick. One group got a sham injection to act as a control group (injected with saline to simulate the feeling of having been injected), one group got a small amount of LPS, and one group got a large amount of LPS. Then, we either gave them an abundance of food or no food. Twenty-four hours after injecting them with LPS, we carefully plucked the spermatophore from the cricket (if it produced one), weighed it, and looked at the amount of sperm that were alive versus dead. The more sperm that were live, the better quality the spermatophore was, indicating that more energy was put into reproduction. If the cricket didn’t produce spermatophore at all, that indicated that the cricket’s body may have allocated less energy towards reproduction and more towards its immune response.
Research Question
What are the effects of food availability and strength of an immune challenge on an organism’s quality of reproductive output?
Methods
Insect Rearing
We bought the crickets from flukerfarms.com and reptilefood.com. They were all raised together in incubators kept at 28-30°C with a 12:12 light cycle, to simulate night and day that they would experience in the real world. The crickets were housed in plastic containers containing empty egg cartons that acted as little houses because the crickets get stressed out if they don’t have a shelter. They were all provided with Purina Cat Chow and water.
Experimental Groups
There were six experimental groups: high LPS, low LPS, or Grace’s Insect Medium (sham) injections, either with food or without food. Each trial consisted of 15 crickets. We made sure the males were capable of reproduction by only using males that already had produced spermatophore. This helped us ensure that the males were adults and able to produce spermatophore for us to analyze. A total of 13 separate trials were done, totaling an experimental group of 195 crickets. The crickets were assigned into their groups using an online randomizer, with even amounts of crickets divided into each injection group. Each trial lasted a span of two days.
Day 1 Experiments
We picked out 15 males that had produced a spermatophore and extracted the spermatophores with a pair of tweezers. After weighing both the crickets and their spermatophores, we injected them in the abdomen with their injection treatments and placed them into their designated deli containers, either with food or without food. We were extra careful when transporting them because, speaking from experience, these critters will take any opportunity to leap out your hands and accidentally jump into a beaker full of ethanol where they will instantaneously die. Once the male crickets were inside their individual containers, they were placed into an incubator that mimicked a natural environment (12:12 night/day cycle at 28-30 degrees celsius).
Day 2 Experiments
After 24 hours, the crickets were checked for spermatophores. If the cricket produced a spermatophore, the mass was recorded. We placed the spermatophore in a live/dead assay solution, which stains them so that under the microscope the dead sperm is red and the live sperm is green. The dead and live sperm were observed and scored to determine sperm viability. Below are photographs we took that show what the sperm will look like under the microscope with the green sperm being alive and the red being dead.
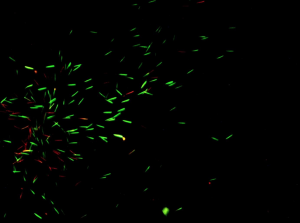
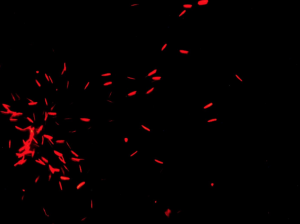
Results
Throughout our experiment, we ran into some major problems as our crickets weren’t producing spermatophore and they were dying at alarming rates. Because our goal was to analyze spermatophore, we had to make a few changes. So, about halfway through, we had to revise our methods including getting crickets from a new place (to try to obtain healthier crickets), raising the temperature the crickets lived at (experiments in our lab had shown that a higher incubation temperature would increase spermatophore production), and enticing them to make a spermatophore by placing female crickets in the container before we put the males in them (having female pheromones in the container may encourage them to make a spermatophore). This created a division in our data between data with our original methods (where crickets weren’t producing spermatophore and dying at alarming rates) and data with the new changes. This means that for every statistical analysis we did, we had three sets of data to look at: all the data without the changes, data before changes were made, and data after changes were made. The divisions of data are important because some had significant data while others did not.
The first variable we investigated was the number of crickets that died after 24 hours in each group. This involved all of the data because we were able to collect this data for every cricket. As you can see from the graph below, the group that had the highest mortality rate was when crickets had a strong immune challenge and the lowest mortality rate was when crickets didn’t have an immune challenge. Another trend that you may notice is that food doesn’t have a large impact on the mortality rate.
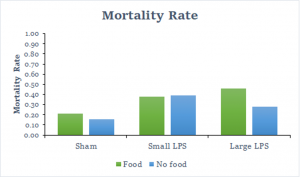
The next finding we explored was how many crickets produced spermatophore compared to how many living crickets there were after 24 hours. The results of this proportion can be seen in the graph below. You can see that there is a noticeable trend here where more spermatophores were produced with the weak immune challenged crickets, but then it drops off significantly with the strong immune challenged crickets. We also only considered the data after we made changes because none of the earlier crickets produced spermatophore.
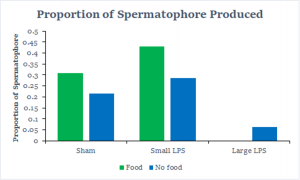
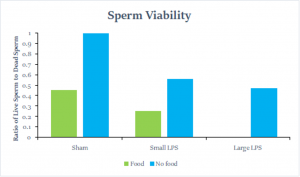
The last, and perhaps the most important finding we had, was concerning sperm viability. We found that both the food availability and the strength of an immune challenge had a great effect on sperm viability. We see that sperm viability decreases as the strength of an immune challenge increases, and that viability was strongest without food. The downside of this is that only 7 spermatophores could be analyzed, so it is hard to draw a strong conclusion.
Interpretation
We found that an immune challenge does cause more crickets to die, and can also increase spermatophore production. This result was partially expected as an immune challenge has been shown to increase mortality rate, but the surprising part is that food didn’t seem to have an impact on mortality rate. This is about like saying whether you’re at a Golden Corral or in the middle of the desert starving, there would be no influence on if you lived or died. Some research has shown that food doesn’t influence death rate, so this research only helps to support that idea.
The spermatophore production was also found to be influenced by an immune challenge when looking at the data after changes were made. The weak immune challenge with no food produced the most amount of spermatophore and the strong immune challenge with no food produced the smallest amount. This finding partially aligns with previous evidence in female A. domesticus that with a stronger immune challenge, egg weight and longevity are significantly lower than with a weaker immune challenge.
The last variable analyzed was the sperm viability of the few spermatophores we had. Overall, we did see that sperm viability decreased with an increase in the strength of an immune challenge. This tells us that a TRI most likely is not made because sperm quality didn’t improve when the crickets may have felt like they were dying. This finding did align with data from other experiments and allowed us to see the clear influence of food availability and strength of an immune challenge. While this is a significant finding, with 0-3 spermatophores per treatment group there is insufficient data to make a definite conclusion.
Significance and Applications
Understanding the trade-offs between the immune and reproductive systems in insects may help us to protect threatened insect species, such as bees. Bees pollinate many of the crops we eat, including apples, blueberries, and cherries. Our fruit salads would look pretty pitiful without these hardworking bees. Unfortunately, bees are threatened by numerous germs and parasites. One of these parasites is called the “vampire mite,” and you can read more about it here. If a farmer noticed that her queen bees were not reproducing well, she might be tempted to give them more food. It would certainly be logical to assume that a queen with more food would have more energy to fight off an infection. However, our research shows that increasing an insect’s food supply does not increase its reproductive output following an immune challenge. Thus, the farmer should try a different approach to increase the size of a colony affected by parasites.

If increasing an insect’s food supply does not affect its reproductive output, then it seems that trade-offs are not necessarily caused by a limited supply of energy. Instead, trade-offs may be caused by a chemical in the insect’s immune system that attacks its own cells, including sperm and eggs. Scientists have pegged a protein called phenoloxidase as a possible perpetrator of this self-destruction. Perhaps we could use this natural insect compound as an alternative to current pesticides. Instead of trying to kill insects (and spraying poison on our crops and termite-ridden basements in the process), we could target their reproduction. By preventing insects from reproducing, we can exterminate pests without introducing harmful chemicals into the environment and our bodies. It’s the difference between using a guided missile and a sledgehammer.
Future Research Directions
Although our research could expand to a host of different directions, we would firstly like to see our experiments redone with healthier crickets. During our trials, we ran into an issue with abnormally high rates of mortality, which likely means that the crickets arrived in a compromised condition. We saw a slight improvement once we changed distributors but due to time constraints, we were unable to obtain a large data set. More viable crickets would hopefully produce spermatophores at a higher rate and have overall lower morality. This would allow us to compare a larger sample of post-treatment spermatophores. Thus, replicating the experiment would test the validity of our findings.
Future research should also examine how food availability and immune challenge strength affect phenoloxidase levels. Phenoloxidase is a chemical produced by an insect’s immune system after a bacterial infection or LPS injection. This immune chemical helps fight off infections, but it can also harm host cells, including sperm and eggs. Even if an insect eats plenty of food to support its reproductive and immune systems, its reproductive cells can still be harmed by phenoloxidase. By studying phenoloxidase, we may figure out why insects invest less in reproduction even when they have access to an all-you-can-eat buffet.
Conclusion
Our research suggests that organisms who activate their immune system spend less energy on reproduction and survival, even if they have access to an all-you-can-eat buffet. This research has broad applications in agriculture, conservation, and pest control. The next time you hear crickets chirping at night, consider how even “simple” creatures have evolved to budget their resources and maximize their reproductive output.
