1
This reading is excerpted from Soderberg, Organic Chemistry with a Biological Emphasis, Ch. 2.3 and McMurry, Organic Chemistry, 10th Ed, Ch. 1.11 and 2.4
Formation of Molecular Orbitals
Molecular orbital (MO) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions) on different atoms to form molecular orbitals, so called because they belong to the entire molecule rather than to an individual atom. Just as an atomic orbital, whether unhybridized or hybridized, describes a region of space around an atom where an electron is likely to be found, so a molecular orbital describes a region of space in a molecule where electrons are most likely to be found.
Like an atomic orbital, a molecular orbital has a specific size, shape, and energy. In the H2 molecule, for example, two singly occupied 1s atomic orbitals combine to form two molecular orbitals. There are two ways for the orbital combination to occur—an additive way and a subtractive way. The additive combination leads to formation of a molecular orbital that is lower in energy and roughly egg-shaped, while the subtractive combination leads to a molecular orbital that is higher in energy and has a node between nuclei:
Note that the additive combination is a single, egg-shaped, molecular orbital; it is not the same as the two overlapping 1s atomic orbitals of the valence bond description. Similarly, the subtractive combination is a single molecular orbital with the shape of an elongated dumbbell. These are referred to as the [latex]\sigma[/latex] bonding molecular orbital (since putting electrons here lead the two atoms to attract each other) and the [latex]\sigma^*[/latex] antibonding molecular orbital (since putting electrons here drive the atoms apart)
When we consider the combination of p orbitals, we need to consider the fact that there are three orthogonal[1] orbitals per p subshell:
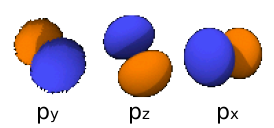
Therefore, there are two ways that these can combine with each other. One of these (typically the [latex]p_x[/latex]) would align along the internuclear axis, and yield [latex]\sigma[/latex] bonding and [latex]\sigma^*[/latex] antibonding molecular orbitals:
What about the other p atomic orbitals? These can combine to form two pairs of bonding and antibonding π molecular orbitals from the sideways combination of two p atomic orbitals in ethylene. The lower-energy, π bonding MO has no node between nuclei and results from the combination of p orbital lobes with the same algebraic sign. The higher-energy, π antibonding MO has a node between nuclei and results from the combination of lobes with opposite algebraic signs.
Resonance and Molecular Orbital Theory
Now, let's get back to resonance. As you may recall, this involves being able to draw two or more Lewis structures for a given molecule, with the arrangement of electrons varying between them. For example, if we consider the nitrite molecule you can draw the following Lewis structures:

To rationalize this, we recognize that the middle N is [latex]sp^2[/latex] hybridized, leaving an unhybridized [latex]p_z[/latex] atomic orbital. This can overlap with the [latex]p_z[/latex] atomic orbitals on the two oxygen atoms to form a delocalized [latex]\pi[/latex] electron cloud. With these three p atomic orbitals, we can form three different delocalized molecular orbitals:
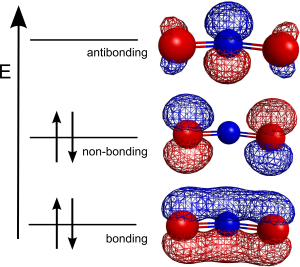
As you can see here, the lowest energy molecular orbital here is a bonding molecular orbital where the electron density is spread out over three atoms. This is what we would call a delocalized [latex]\pi[/latex] bond, and explains how we can form delocalized bonds when there are resonance structures.
What these resonance structures are telling us is that instead of there being a pi bond between the N and just one of the O’s in the nitrate ion, that pi bond is actually spread out over all 3 atoms, meaning that instead of having one N=O double bond and one N-O single bond, there are actually 2 one-and-a-half N-O bonds. This can also be seen by looking at the bonding orbital of the molecular orbital diagram of nitrite below, where you can see that the pi bond extends over all 3 atoms of nitrite. The resonance structures above also tell us that both O’s share the 3rd lone pair and thus the negative charge, meaning that instead of 1 neutral O and 1 negatively charged O, nitrate actually has 2 O’s with a -0.5 charge. This can also be seen in the non-bonding orbital of nitrate’s molecular orbital diagram below where we can see that the non-bonding electrons (the lone pair) can spend time on both of the O’s, but because there is a node on the N in the middle, that N does not see these electrons.
Benzene
A particularly important example to consider is benzene, the simplest aromatic compound, for which we can draw two equivalent resonance forms.
Although benzene is most often drawn with three double bonds and three single bonds, in fact all of the carbon-carbon bonds are exactly the same length (138 pm). In addition, the pi bonds in benzene are significantly less reactive than 'normal' pi bonds, either isolated or conjugated. Something about the structure of benzene makes its pi bonding arrangement especially stable. This ‘something’ has a name: it is called ‘aromaticity’.
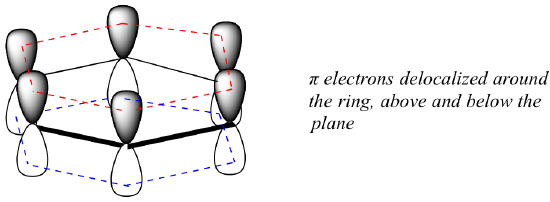
What exactly is this ‘aromatic’ property that makes the pi bonds in benzene so stable? In large part, the answer to this question lies in the fact that benzene is a cyclic molecule in which all of the ring atoms are sp2-hybridized. This allows the pi electrons to be delocalized in molecular orbitals that extend all the way around the ring, above and below the plane. For this to happen, of course, the ring must be planar – otherwise the p orbitals couldn’t overlap properly. Benzene is indeed known to be a flat molecule.
- At right angles to each other ↵

