2.1 Air pollution – overview and “traditional” air pollutants
meretsky
Air pollution occurs in many forms but can generally be thought of as gaseous and particulate contaminants that are present in the earth’s atmosphere. Chemicals discharged into the air that have a direct impact on the environment are called primary pollutants. These primary pollutants sometimes react with other chemicals in the air to produce secondary pollutants.
Air pollution takes the form of aerosols and gases. Aerosols are made up of liquid and solid particles , sometimes called particulates, suspended in the atmosphere such as soot, dust, and volcanic ash. Air-polluting gases include carbon dioxide (CO2), sulfur dioxide (SO2), a variety of nitrogen oxides (mostly NO and NO2, cumulatively written as NOx) and ozone (O3). The components of air pollution undergo chemical reactions in the atmosphere that can generate additional pollutants. For example, sulfur dioxide gas can dissolve in water droplets to create an aerosol of sulfuric acid droplets that contributes to acid rain. Reactions of VOCs and NOx can lead to the formation of O3 and aerosols, the primary components of photochemical smog. Chemical changes are driven by energy from sunlight that helps to produce highly reactive chemical components such as ozone.
Outdoor and indoor air pollution are usually described separately. Outdoor air pollution involves exposures that take place outside of the built environment. Examples include fine particles produced by coal burning, noxious gases such as sulfur dioxide, nitrogen oxides, carbon monoxide, and ground-level ozone. Indoor air pollution involves exposure to particulates, carbon oxides, and other indoor air or dust pollutants. Examples include household products and chemicals, out-gassing of building materials, allergens (cockroach and mouse dropping, mold, pollen), and tobacco smoke.
Sources of air pollution
A stationary source of air pollution refers to an emission source that does not move, also known as a point source. Stationary sources include factories and power plants. The term area source describes many small, dispersed sources of air pollution located together whose individual emissions may be below thresholds of concern but whose collective emissions can be significant. Residential wood burners, gas stations, and dry cleaners are good examples of small sources; when they occur at high densities, they can contribute to local and regional air pollution levels. Area sources of air pollution are considered to be nonpoint sources.
A mobile source of air pollution refers to a source capable of moving under its own power. Mobile sources generally imply “on-road” transportation, including cars, sport utility vehicles, and buses. In addition, there is also a “non-road” or “off-road” category that includes gas-powered lawn tools and mowers, farm and construction equipment, recreational vehicles, boats, planes, and trains.
Agricultural sources arise from operations that raise animals and grow crops, which can generate emissions of gases and particulate matter. For example, animals confined to a barn or restricted area produce large amounts of manure. Manure emits various gases, particularly ammonia and methane, into the air. These gases can be emitted from animal houses, manure storage areas, or land after the manure is applied. In crop production, the misapplication of fertilizers, herbicides, and pesticides can potentially result in aerial drift of these materials, and harm may be caused. We will see manure as a source of water pollution, as well, in a later chapter.
Unlike the anthropogenic sources of air pollution described above, air pollution caused by natural sources is not caused by people or their activities. An erupting volcano emits particulate matter and gases, forest, and prairie fires can emit large quantities of “pollutants,” dust storms can create large amounts of particulate matter, and plants and trees naturally emit volatile organic compounds which can form aerosols that can cause a natural blue haze. Wild animals in their natural habitats are also considered natural sources of “pollution” in the form of methane, urine and manure.
Air pollutants regulated in the US
In the US, the six most common outdoor air pollutants during the 1970s were targeted for clean-up in the Clean Air Act: particulate matter, ground-level ozone, carbon monoxide, sulfur oxides, nitrogen oxides, and lead. These are the pollutants classed here as “traditional” air pollutants because they are have been the subject of the longest-running efforts at control and regulation in the US and internationally. In the US they are referred to as criteria pollutants because the EPA sets criteria to control their use. These pollutants can harm health and the environment and cause property damage. At present, of the six criteria pollutants, particulates and ground-level ozone are the most widespread health threats. The U.S. Environmental Protection Agency (EPA) regulates them by developing criteria based on human and environmental health considerations. Sources for major air pollutants in the US are shown in Figure 2, below. Greenhouse gases (GHG) which cause global warming and climate change are regulated similarly to more traditional air pollutants, but are controlled and regulated differently. They are addressed in a later section of this chapter.
Ground-level ozone is not emitted directly into the air but is created by chemical reactions in smog between nitrogen oxides (NOx) and volatile organic compounds (VOCs), in the presence of sunlight. Emissions from industrial facilities and electric utilities, motor vehicle exhaust, gasoline vapors, and chemical solvents are some of the major sources of NOx and VOCs. Breathing ozone can trigger various health problems, particularly for children, the elderly, and people of all ages with lung diseases such as asthma. Ground-level ozone can also have harmful effects on vegetation and ecosystems and can reduce crop production. (Ground-level ozone should not be confused with the ozone layer, which is high in the atmosphere and protects Earth from ultraviolet light; ground-level ozone provides no such protection).
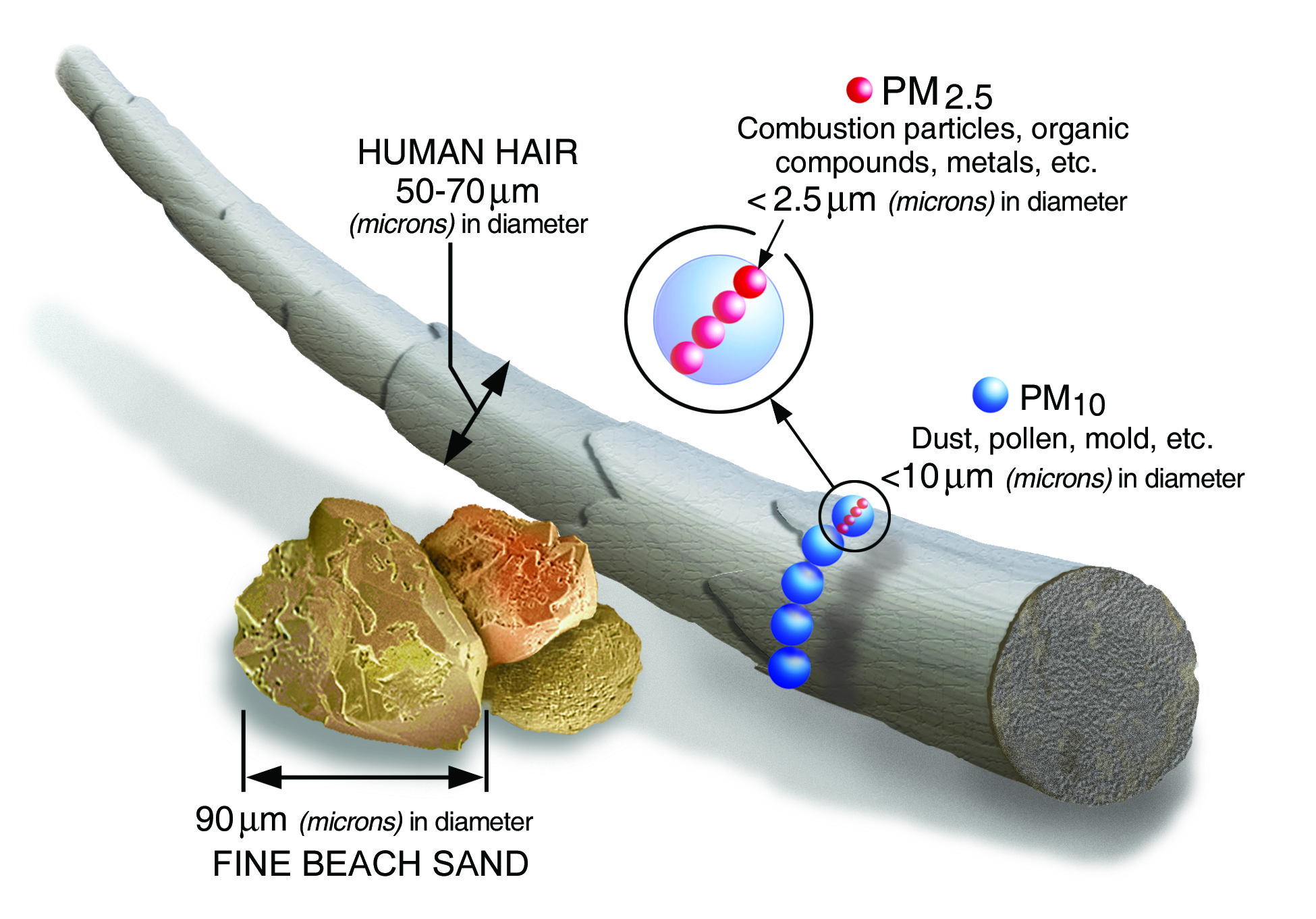
Particulate matter, also known as particle pollution, comprises extremely small particles and liquid droplets. Particle pollution comprises several components, including acids (such as nitrates and
sulfates), organic chemicals, metals, and soil or dust particles. The size of particles is directly linked to their potential to cause health problems. EPA is concerned about particles that are 10 micrometers in diameter or smaller because those are the particles that generally pass through the throat and nose and enter the lungs. Once inhaled, these particles can affect the heart and lungs and cause serious health effects. The most harmful are less than 2.5 micrometers in diameter (human hairs range from 50-180 micrometers in diameter – Fig 1); some particles that enter the lungs can enter the bloodstream.
Carbon monoxide (CO) is a colorless, odorless gas emitted from combustion processes. Nationally, and, particularly in urban areas, the majority of outdoor CO emissions come from mobile sources. CO can cause harmful health effects by reducing oxygen delivery to the body’s organs (like the heart and brain) and tissues. At extremely high levels, CO can cause death.
Nitrogen dioxide (NO2) is one of a group of highly reactive gases known as “oxides of nitrogen,” or nitrogen oxides (NOx). Other nitrogen oxides include nitrous acid and nitric acid. EPA’s National Ambient Air Quality Standard uses NO2 as the indicator for the larger group of nitrogen oxides. NO2 forms quickly from emissions from cars, trucks, buses, power plants, and off-road equipment. In addition to contributing to the formation of ground-level ozone, and fine particle pollution, NO2 is linked with a number of adverse effects on the respiratory system.
Sulfur dioxide (SO2) is one of a group of highly reactive gases known as “oxides of sulfur.” In 2020, the largest sources of SO2 emissions were from coal combustion at power plants (48%) and emissions from industrial facilities (19%). Smaller sources of SO2 emissions include industrial processes such as extracting metal from ore and burning high sulfur-containing fuels by locomotives, large ships, and non-road equipment. SO2 is linked with a number of adverse effects on the respiratory system.
Lead is a metal found naturally in the environment and manufactured products. The major sources of lead emissions, historically, were fuels for on-road motor vehicles (such as cars and trucks) and industrial sources. As a result of regulatory efforts in the U.S. to remove lead from on-road motor vehicle gasoline (it had been used as an additive to improve engine performance), emissions of lead from the transportation sector declined by 95% between 1980 and 1999, and levels of lead in the air decreased by 94% between 1980 and 1999. Internationally, all countries have banned lead as an additive to gasoline, since 2021. Today, the highest lead levels in the air are usually found near lead smelters; today’s major sources of lead emissions to the air are ore and metals processing and old-fashioned aircraft that still use leaded aviation gasoline. Incinerators used to process solid waste and lead-acid battery manufacturers are other significant sources. [1]
In addition to these criteria pollutants, the US regulates radioactive and toxic (or hazardous) air pollutants, pollutants that affect the protective ozone layer in the stratosphere that protects living things from harmful UV radiation from the sun, and CO2 emissions that contribute to global warming. Toxic air pollutants including many chemicals used in industry, asbestos, and heavy metals such as lead, mercury, cadmium, and chromium; they cause a variety of harms to health including neurological reproductive, developmental, respiratory and cardiac problems.
Many toxic air pollutants can settle out of the atmosphere or rain out onto the soil or into the water, where they can be absorbed to soil, or taken in by drinking (animals) or root uptake (plant). Pollutants, particularly persistent organic pollutants that have long lifetimes, can travel long distances in the air before finally settling and raining out – termed long-range transport. Because planetary winds tend to spiral towards the poles, pollutant levels on the ground and in the water near the poles can be unexpectedly high, despite being far from industry or human settlement.
Pollutants that affect the ozone layer are often chemicals in refrigerants, foams, aerosols, solvents, and fire suppressants. By reducing ozone in the stratosphere, they increase UV radiation that can cause skin cancer, harm vegetation, and decrease crop production.
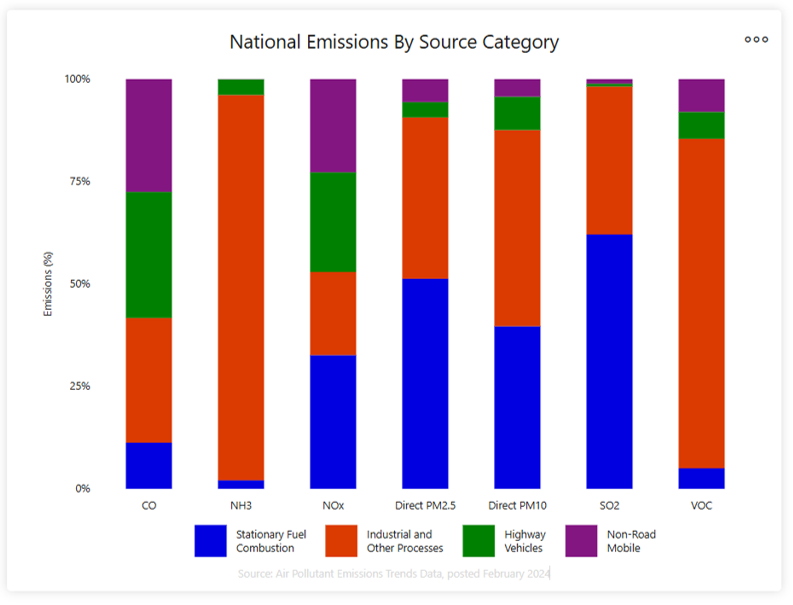
Generally, emissions of air pollution come from
- stationary fuel combustion sources (such as electric utilities and industrial boilers)
- industrial and other processes (such as metal smelters, petroleum refineries, cement kilns and dry cleaners)
- highway vehicles
- non-road mobile sources (such as recreational and construction equipment, marine vessels, aircraft and locomotives)
Acid rain
The primary contributors to acid rain are SO2 and NOx. Acid rain harms building materials, especially marble and limestone that dissolve in acid water, metals, and paints. In ecosystems, acid rain harms vegetation and can kill it if exposure is severe and prolonged. Acid rain acidifies soil and water, releasing aluminum from soils and substrates; at pH levels below approximately 5.0, fish and most invertebrates in soil and water cannot persist due to aluminum toxicity. In areas where soils contain neutralizing, buffering materials such as calcium carbonate from limestone, soil and water acidity are lessened, but the process depletes the soils of the buffering materials. At the height of acid rain, in the 1980s, entire forests in Europe were killed (Fig 3). In the northeastern US and Canada, where acid rain was severe due to both local and upwind burning of fossil fuels, and where granite soils have only low concentrations of buffering materials, most lakes became “dead,” devoid of fish, invertebrates, and even algae. With the lower levels of the food web eliminated, higher-level predators that relied on aquatic foods, such as osprey, a fish-eating bird of prey, also disappeared. In these lakes, with little buffering material to neutralize acid waters, pH levels are recovering very slowly, and buffering materials remain reduced. However, due to chemistry involving plants, toxic aluminum concentrations are declining more quickly. Fish are returning to some lakes.
Acid rain can also harm human health if people are breathing air with acid aerosols – usually in polluted urban environments, during rain events. Eye and throat irritation and damage to lungs can occur. Asthma and other respiratory conditions and some heart conditions may be exacerbated. This is just an extension of air pollution health effects – SO2 and NOx pollution can cause these problems in the absence of rain, too.
International air pollutants of note
The World Health Organization (WHO) provides guidelines for all of the 6 US criteria pollutants, plus three major indoor air pollutants: polycyclic aromatic hydrocarbons (PAHs – like carbon monoxide, these are mostly formed by incomplete combustion of fossil fuels and natural fuels), formaldehyde (a common indoor air pollutant and a VOC), radon (a radioactive gas and indoor air pollutant), In addition, the WHO provides good practice statements, but not guidelines, for three additional air pollutants. Black carbon, also known as soot, is an important indoor and outdoor air pollutant in countries where household fires are still a common means of heating and cooking and where diesel fuels are burned without accompanying pollution controls. Ultrafine particles (PM0.1 – particles less than 0.1 micrometers in diameter) mostly result from incomplete burning of fuels in transportation, power generation, and heating; they increase the risk for heart and lung diseases. Molds are fungi that can be allergens and irritants in indoor air, usually resulting from poor ventilation and moisture build-up; they can cause respiratory damage including asthma attacks.
Indoor air pollution
Most people spend approximately 90% of their time indoors. However, the indoor air we breathe in homes and other buildings can be more polluted than outdoor air and can increase the risk of illness. There are many sources of indoor air pollution in homes. They include biological contaminants such as bacteria, molds, and pollen, burning fuels and environmental tobacco smoke, chemicals, including volatile organic compounds, in building materials and furnishings, household products, central heating and cooling systems, and outdoor sources. Outdoor air pollution can enter buildings and become a source of indoor air pollution. Where cooking and heating use diesel and other dirtier fossil fuels, manure, wood, or other biomass, indoor air pollution can also include soot and higher levels of carbon monoxide.
Sick building syndrome is a term used to describe situations where building occupants have health symptoms associated only with spending time in that building. Causes of sick building syndrome include inadequate ventilation, indoor air pollution, and biological contaminants. Usually, indoor air quality problems only cause discomfort. Most people feel better as soon as they remove the source of the pollution.
Natural sources of air pollution
Many air pollutants occur naturally in measurable quantities. Wildfires, in particular, create many problematical substances, including CO2, CO, CH4, NOx, VOC (recall that NOx and VOC can be precursors to ground-level ozone) and particulates including black carbon. Wind-borne dust from deserts, rangelands and degraded agricultural lands can contribute to very high levels of particulates. Trees emit VOCs that can contribute to ground-level ozone. Volcanoes produce a wide array of substances including all of the traditional air pollutants, plus heavy metals. The term “vog” is used to describe the combination of gases and particulates produced by volcanoes, which can be hazardous to human and environmental health and harmful to property. SO2 is usually a substantial component of volcanic gas and can contribute to very acidic aerosols; volcanic particulates include fine, very sharp particles of volcanic glass that can damage lungs, skin, plants, and equipment.
Knowledge Check:
Take a moment to complete the short quiz below to assess your understanding of this section. Read each question carefully and refer back to the content as needed. This quiz is not graded – it’s simply an opportunity for you to reflect on what you’ve learned and reinforce key concepts.
Media Attributions
- pm2.5_scale_graphic-color_2.EPA2016-09 © US EPA is licensed under a Public Domain license
- US emissions by source.EPA24 report © US EPA is licensed under a Public Domain license
- Effects of acid rain on forest in Czech mountains.2006.brightened © Modified from public domain adapted by Vicky Meretsky is licensed under a Public Domain license
- EPA. 2025. Basic information about lead air pollution. US Environmental Protection Agency. https://www.epa.gov/lead-air-pollution/basic-information-about-lead-air-pollution#how ↵