1.5 Classification of Matter (Element, Compound, Mixture)
Joey Wu and OpenStax
Learning Objectives
- Distinguish pure substances from mixtures.
- Distinguish elements from compounds.
- Distinguish homogeneous mixture from heterogeneous mixtures.
- Describe molecule
Molecule
It is rare to find collections of individual atoms. Only a few elements, such as the gases helium, neon, and argon, consist of a collection of individual atoms that move about independently of one another. Other elements, such as the gases hydrogen, nitrogen, oxygen, and chlorine, are composed of units that consist of pairs of atoms (Figure 1). One form of the element phosphorus consists of units composed of four phosphorus atoms. These units are called molecules. A molecule consists of two or more atoms joined by strong forces called chemical bonds. The atoms in a molecule move around as a unit, much like the cans of soda in a six-pack or a bunch of keys joined together on a single key ring.
A molecule may consist of two or more identical atoms, as in the molecules found in the elements hydrogen, oxygen, and sulfur, or it may consist of two or more different atoms, as in the molecules found in water. Each water molecule is a unit that contains two hydrogen atoms and one oxygen atom. Each glucose molecule is a unit that contains 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. Like atoms, molecules are incredibly small and light. If an ordinary glass of water were enlarged to the size of the earth, the water molecules inside it would be about the size of golf balls.
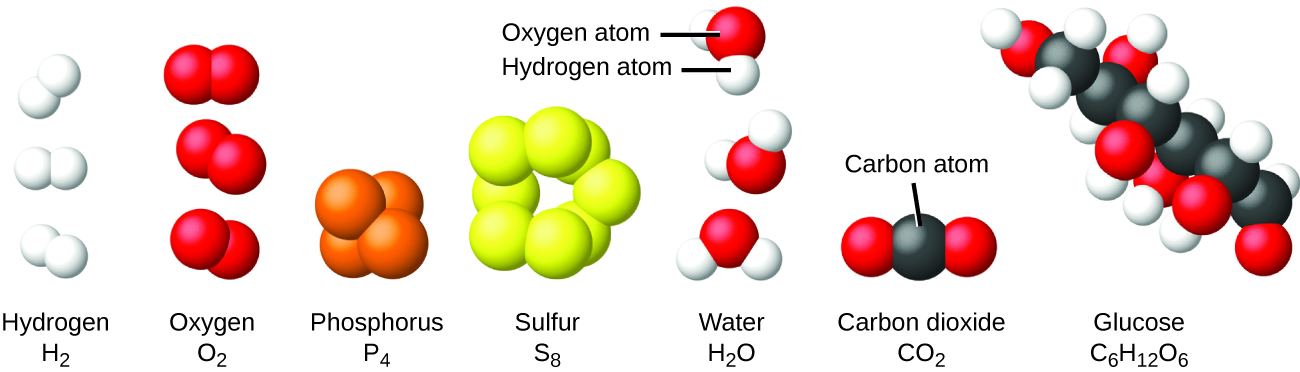
Figure 1. The elements hydrogen, oxygen, phosphorus, and sulfur form molecules consisting of two or more atoms of the same element. The compounds water, carbon dioxide, and glucose consist of combinations of atoms of different elements.
Classifying Matter
Matter can be classified into several categories. Two broad categories are mixtures and pure substances. A pure substance has a constant composition. Any pieces of a pure substance have exactly the same makeup and properties. For example, any sample of sucrose (table sugar; Figure 2) consists of 42.1% carbon, 6.5% hydrogen, and 51.4% oxygen by mass. They also has the same physical properties, such as melting point, color, and sweetness, regardless of the source from which it is isolated.

Figure 2. All sucrose (table sugar) have the same composition of elements and physical properties.
Elements vs. Compounds
Pure substances may be divided into two classes: elements and compounds. Pure substances that cannot be broken down into simpler substances by chemical changes are called elements. Iron, silver, gold, aluminum, sulfur, oxygen, and copper are familiar examples of the more than 100 known elements, of which about 90 occur naturally on the earth, and two dozen or so have been created in laboratories. Elements exist everywhere in our life. For example, Neon is often used in colored lights (Figure 3).

Figure 3. The red lights in this sign contain the element Neon
Pure substances that are comprised of two or more elements are called compounds. Compounds may be broken down by chemical changes to yield either elements or other compounds, or both. For example, mercury(II) oxide, an orange, crystalline solid, can be broken down by heat into the elements mercury and oxygen (Figure 4). Silver(I) chloride is a white solid that can be broken down into its elements, silver and chlorine, by absorption of light. This property is the basis for the use of this compound in photographic films and photochromic eyeglasses (those with lenses that darken when exposed to light).
Link to Learning
Many compounds break down when heated. This site shows the breakdown of mercury oxide, HgO. You can also view an example of the photochemical decomposition of silver chloride (AgCl), the basis of early photography.
Mixtures
A mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by physical changes, such as evaporation (you will learn more about this later). A mixture with a composition that varies from point to point is called a heterogeneous mixture. Italian dressing is an example of a heterogeneous mixture (Figure 4). Its composition can vary because it may be prepared from varying amounts of oil, vinegar, and herbs. It is not the same from point to point throughout the mixture—one drop may be mostly vinegar, whereas a different drop may be mostly oil or herbs because the oil and vinegar separate and the herbs settle.
A homogeneous mixture exhibits a uniform composition and appears visually the same throughout. An example of a solution is a sports drink, consisting of water, sugar, coloring, flavoring, and electrolytes mixed together uniformly (Figure 5). Each drop of a sports drink tastes the same because each drop contains the same amounts of water, sugar, and other components. Note that the composition of a sports drink can vary—it could be made with somewhat more or less sugar, flavoring, or other components, and still be a sports drink. Other examples of homogeneous mixtures include air, maple syrup, gasoline, and a solution of salt in water.
Elements on Earth
Eleven elements make up about 99% of the earth’s crust and atmosphere (Table 1). Oxygen constitutes nearly one-half and silicon about one-quarter of the total quantity of these elements. A majority of elements on earth are found in chemical combinations with other elements; about one-quarter of the elements are also found in the free state.
| Elemental Composition of Earth | ||||||
|---|---|---|---|---|---|---|
| Element | Symbol | Percent Mass | Element | Symbol | Percent Mass | |
| oxygen | O | 49.20 | chlorine | Cl | 0.19 | |
| silicon | Si | 25.67 | phosphorus | P | 0.11 | |
| aluminum | Al | 7.50 | manganese | Mn | 0.09 | |
| iron | Fe | 4.71 | carbon | C | 0.08 | |
| calcium | Ca | 3.39 | sulfur | S | 0.06 | |
| sodium | Na | 2.63 | barium | Ba | 0.04 | |
| potassium | K | 2.40 | nitrogen | N | 0.03 | |
| magnesium | Mg | 1.93 | fluorine | F | 0.03 | |
| hydrogen | H | 0.87 | strontium | Sr | 0.02 | |
| titanium | Ti | 0.58 | all others | – | 0.47 | |
Table 1. Elemental Composition of Earth
Exercises
Classify each of the following as an element, a compound, or a mixture:
- (a) copper
- (b) water
- (c) nitrogen
- (d) sulfur
- (e) air
- (f) sucrose
- (g) a substance composed of molecules each of which contains two iodine atoms
- (h) gasoline
- (a) iron
- (b) oxygen
Free access via Pressbook
Media Attributions
- table sugar
- open sign

