1.2 Atom and Atomic Theory
Joey Wu and Adam Maltese
Learning Objectives
- Understand that atoms are the building blocks of matters
- Learn how atoms are constructed.
- Describe basic characteristics of three subatomic particles
Atoms
In many science subjects, you usually have to become quite advanced before you can start discussing the more thought-provoking philosophical ideas. However, in chemistry, things are a bit different. We dive right in to explore one of the most philosophically intriguing aspects of the entire subject: the atom.

Figure 1. Ancient Greek Philosopher, Democritus, known as the “laughing philosopher”.
About 2000 years ago, a Greek philosopher Democritus (Figure 1) wondered, for example, what would happen if you cut a chunk of matter — such as an apple into smaller pieces (Figure 2). He thought that a point would be reached at which the apple could not be cut into still smaller pieces, and still be an apple. He called these pieces atomos, a term derived from the Greek word for “indivisible” or “uncuttable”. This is where the modern term atom comes from.

Figure 2. If we keep cutting an apple to the smallest pieces, what would happen?
In truth, it does not have to be an apple. This is true for any substances or any elements that you encounter in the universe. We now know that the smallest piece that maintains the property of the substance/elements is called an atom. It is not an indivisible solid like Democritus claimed, but consists of smaller particles.
Democritus was an Atomist and this group held that everything is composed of “atoms,” which are not physically separable and are indestructible. Atoms exist in a void or empty space and have been and will forever continue to be in motion. Additionally, they believed there is an infinite number of atoms, but that these vary in shape and size. They also believed that atoms had hooks, sockets and other parts that allowed them to connect with other atoms and this is how different materials were created. Learn more here.
Size of Atoms
Atoms are the building blocks of matter. Individual atoms are extremely small. It would take about fifty million atoms in a row to make a line that is 1 cm long. The period at the end of a printed sentence has several million atoms in it. Atoms are so small that it is difficult to believe that all matter is made from atoms—but it is.
Learn about the power of ten via the following video:
Modern Atomic Theory

Figure 3. Chemist John Dalton is credited with pioneering modern atomic theory. He was also the first to study color blindness
The concept that atoms play a fundamental role in chemistry was formalized by the modern atomic theory, first stated by John Dalton (Figure 3), an English scientist, in 1808. It consists of three parts:
- All matter is composed of atoms.
- Atoms of the same element are the same; atoms of different elements are different.
- Atoms combine in whole-number ratios to form compounds.
These concepts form the basis of chemistry.
Subatomic Particles
Although the word atom comes from a Greek word that means “indivisible,” we understand now that atoms themselves are composed of smaller parts called subatomic particles. The first part to be discovered was the electron, a tiny subatomic particle with a negative charge. It is often represented as e−, with the right superscript showing the negative charge.
Later, two larger particles were discovered — proton and neutron. The proton is a more massive (but still tiny) subatomic particle with a positive charge, represented as p+. The neutron is a subatomic particle with about the same mass as a proton but no charge. It is represented as either n or n0. We now know that all atoms of all elements are composed of electrons, protons, and (with one exception) neutrons. Table 1 “Properties of the Three Subatomic Particles” summarizes the properties of these three subatomic particles.
| Name | Symbol | Mass (approx.; kg) | Charge |
|---|---|---|---|
| Proton | p+ | 1.6 × 10−27 | 1+ |
| Neutron | n, n0 | 1.6 × 10−27 | none |
| Electron | e− | 9.1 × 10−31 | 1− |
Table 1. Properties of the Three Subatomic Particles
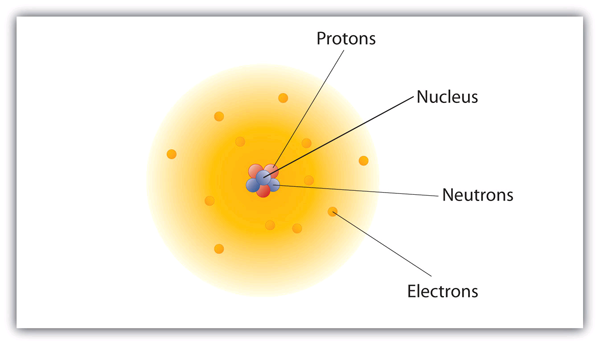
How are these particles arranged in atoms? They are not arranged at random. Experiments by Ernest Rutherford in England in the 1910s pointed to a nuclear model of the atom. The relatively massive protons and neutrons are collected in the center of an atom, in a region called the nucleus of the atom (plural nuclei). The electrons are outside the nucleus and spend their time orbiting in space about the nucleus. (See Figure 4 “The Structure of the Atom”.)
Figure 4. The Structure of the Atom
Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus.
Key Takeaways
- Atoms are the smallest piece that maintains the properties of any substance/elements.
- Chemistry is based on the modern atomic theory, which states that atoms are the building blocks of all matter.
- Atoms themselves are composed of protons, neutrons, and electrons.
Exercises
-
List the three statements that make up the modern atomic theory.
-
Explain how atoms are composed.
-
Which is larger, a proton or an electron?
-
Which is larger, a neutron or an electron?
-
What are the charges for each of the three subatomic particles?
-
Where is most of the mass of an atom located?
Media Attributions
- democritus
- apple_01
- john dalton