1.4 Isotopes and Elements
Joey Wu and Adam Maltese
Atomic Number
The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. What makes atoms of different elements different? The fundamental characteristic that all atoms of the same element share is the number of protons.
For example, all atoms of hydrogen have one and only one proton in the nucleus; all atoms of iron have 26 protons in the nucleus. This number of protons is so important to the identity of an atom that it is called the atomic number of the element. Thus, hydrogen has an atomic number of 1, while iron has an atomic number of 26. Each element has its own characteristic atomic number.
Isotopes
Atoms of the same element can have different numbers of neutrons, however. Atoms of the same element (i.e., atoms with the same number of protons) with different numbers of neutrons are called isotopes. Most naturally occurring elements exist as isotopes. For example, most hydrogen atoms have a single proton in their nucleus. However, a small number (about one in a million) of hydrogen atoms have a proton and a neutron in their nuclei. This particular isotope of hydrogen is called deuterium. A very rare form of hydrogen has one proton and two neutrons in the nucleus; this isotope of hydrogen is called tritium. The sum of the number of protons and neutrons in the nucleus is called the mass number of the isotope.
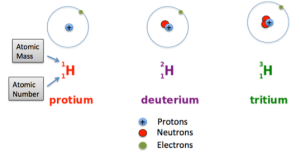
Figure 1. Three isotopes of hydrogen element: Protium, Deuterium, and Tritium. The only difference between them is the number of neutrons.
Different isotopes of an element generally have the same physical and chemical properties. That’s because they have the same numbers of protons and electrons.
Example 1
An isotope of uranium has an atomic number of 92 and a mass number of 235. What are the number of protons and neutrons in the nucleus of this atom?
Solution
If the atomic number of uranium is 92, then that is the number of protons in the nucleus. Because the mass number is 235, then the number of neutrons in the nucleus is 235 − 92, or 143.
Stability of Isotopes
Atoms need a certain ratio of neutrons to protons to have a stable nucleus. Having too many or too few neutrons relative to protons results in an unstable, or radioactive, nucleus that will sooner or later break down to a more stable form. This process is called radioactive decay. Many isotopes have radioactive nuclei, and these isotopes are referred to as radioisotopes. When they decay, they release particles that may be harmful. This is why radioactive isotopes are dangerous and why working with them requires special suits for protection. The isotope of carbon known as carbon-14 is an example of a radioisotope. In contrast, the carbon isotopes called carbon-12 and carbon-13 are stable.
Elements
When referring to an atom, we simply use the element’s name: the term sodium refers to the element as well as an atom of sodium. But it can be unwieldy to use the name of elements all the time. Instead, chemistry defines a symbol for each element. The atomic symbol is a one- or two-letter abbreviation of the name of the element. By convention, the first letter of an element’s symbol is always capitalized, while the second letter (if present) is lowercase. Thus, the symbol for hydrogen is H, the symbol for sodium is Na, and the symbol for nickel is Ni. Most symbols come from the English name of the element, although some symbols come from an element’s Latin name. (The symbol for sodium, Na, comes from its Latin name, natrium.) Table 2 “Names and Symbols of Common Elements” lists some common elements and their symbols. We will talk more about elements and Periodic Table in the next few chapters.
| Element Name | Symbol | Element Name | Symbol | |
|---|---|---|---|---|
| Aluminum | Al | Mercury | Hg | |
| Argon | Ar | Molybdenum | Mo | |
| Arsenic | As | Neon | Ne | |
| Barium | Ba | Nickel | Ni | |
| Beryllium | Be | Nitrogen | N | |
| Bismuth | Bi | Oxygen | O | |
| Boron | B | Palladium | Pd | |
| Bromine | Br | Phosphorus | P | |
| Calcium | Ca | Platinum | Pt | |
| Carbon | C | Potassium | K | |
| Chlorine | Cl | Radium | Ra | |
| Chromium | Cr | Radon | Rn | |
| Cobalt | Co | Rubidium | Rb | |
| Copper | Cu | Scandium | Sc | |
| Fluorine | F | Selenium | Se | |
| Gallium | Ga | Silicon | Si | |
| Germanium | Ge | Silver | Ag | |
| Gold | Au | Sodium | Na | |
| Helium | He | Strontium | Sr | |
| Hydrogen | H | Sulfur | S | |
| Iodine | I | Tantalum | Ta | |
| Iridium | Ir | Tin | Sn | |
| Iron | Fe | Titanium | Ti | |
| Krypton | Kr | Tungsten | W | |
| Lead | Pb | Uranium | U | |
| Lithium | Li | Xenon | Xe | |
| Magnesium | Mg | Zinc | Zn | |
| Manganese | Mn | Zirconium | Zr |
Table 2. Names and Symbols of Common Elements
There is an easy way to represent isotopes using the atomic symbols. We use the construction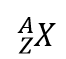
where X is the symbol of the element, A is the mass number, and Z is the atomic number. Thus, for the isotope of carbon that has 6 protons and 6 neutrons, the symbol is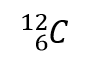
where C is the symbol for the element, 6 represents the atomic number, and 12 represents the mass number.
Example 2
- What is the symbol for an isotope of uranium that has an atomic number of 92 and a mass number of 235?
- How many protons and neutrons are in 26Fe?
Solution
- The symbol for this isotope is 92U.
- This iron atom has 26 protons and 56 − 26 = 30 neutrons.
Test Yourself
How many protons are in 11N?
Answer
11 protons
It is also common to state the mass number after the name of an element to indicate a particular isotope. Carbon-12 represents an isotope of carbon with 6 protons and 6 neutrons, while uranium-238 is an isotope of uranium that has 146 neutrons.
Key Takeaways
- Atoms of the same element that differ in their numbers of neutrons are called isotopes. Different isotopes of an element generally have the same physical and chemical properties because they have the same numbers of protons and electrons.
- Atoms need a certain ratio of neutrons to protons to have a stable nucleus. If they have too many or too few neutrons relative to protons, they are radioactive and will decay to more stable forms.
- Elements are represented by an atomic symbol.
- The periodic table is a chart that organizes all the elements.
Exercises
-
Define atomic number.
-
What is the atomic number of helium?
-
Define isotope and give an example.
-
What is the difference between deuterium and tritium?
-
Which pair represents isotopes?
-
Which pair represents isotopes?
-
a) 20Ca and 19K
-
b) 19F and 18F
-
c) 92U and 92U
-
-
Give complete symbols of each atom, including the atomic number and the mass number (You can find the symbol from Table 2)
-
a) an oxygen atom with 8 protons and 8 neutrons
-
b) a potassium atom with 19 protons and 20 neutrons
-
c) a lithium atom with 3 protons and 4 neutrons
-
-
Give complete symbols of each atom, including the atomic number and the mass number (You can find the symbol from Table 2)
-
a) a magnesium atom with 12 protons and 12 neutrons
-
b) a magnesium atom with 12 protons and 13 neutrons
- c) a boron atom with 5 protons and 6 neutrons
-
-
Carbon-14 is an isotope used to perform radioactive dating tests on previously living material. Its atomic number is 6. What is the complete symbol for this isotope?
Media Attributions
- hydrogen isotope
- Ionic-Compounds-1
- Ruler-1

