Part One: Fundamentals of Environmental Geology
Lab 3 Reading: Minerals
Have you used a mineral yet today? While many people may initially say no, answer these questions: Have you brushed your teeth? Have you eaten anything that might contain salt? Did you put on make-up this morning, or do you have painted fingernails or toenails? Have you used a cellphone? What about a car, bike, or public transportation? If you have done any of those things, you have used at least one mineral, and in many cases you have used a great number of minerals. Minerals are very useful and common in everyday products, but most people do not even realize it.
A mineral is defined as a naturally occurring, inorganic solid with a definite chemical composition and a characteristic crystalline structure. Let’s break that definition down. By naturally occurring, it means that anything man has created, like the beautiful synthetic bismuth in Figure 1, does not count as a mineral. To be an inorganic solid, the mineral must not be composed of the complex carbon molecules that are characteristic of life and must be in the solid state, rather than vapor or liquid. This means that water, a liquid, is not a mineral, while ice, a solid, would be (as long as it is not man-made). A definite chemical composition refers to the chemical formula of a mineral. For most minerals, this does not vary (ex. halite is Na- Cl), though some minerals have a range of compositions, since one element can substitute for another of similar size and charge (ex. olivine is (Mg,Fe)2SiO4, and its magnesium and iron content can vary). The atoms within minerals are lined up in an orderly fashion, so that the characteristic crystalline structure is just an outward manifestation of the internal atomic arrangement.

Figure 1 | Synthetic bismuth
Author: Philippe Giabbanelli Source: Wikimedia Commons License: CC BY-SA 3.0
Minerals are not only important for their many uses, but also as the building blocks of rocks. In this lab, you will lay the foundation for all the future rock labs in the course. Correct mineral identification is critical in geology, so work through this lab carefully. There are several thousand minerals, but we will focus on only eighteen of the most common ones.
Learning Objectives
After completing this chapter, you should be able to:
- Know the definition of a mineral
- Understand the many different physical properties of minerals, and how to apply them to mineral identification
- Be able to distinguish mineral cleavage from mineral fracture
- Identify 18 minerals
Key Terms
- Cleavage
- Crystal Form
- Fracture
- Hardness
- Luster
- Mineral
- Specific Gravity
- Streak
- Tenacity
PHYSICAL PROPERTIES
Identifying a mineral is a little like playing detective. Minerals are identified by their physical properties. For example, look at Figure 2. How would you describe it? You may say that it is shiny, gold, and has a particular shape. Each of these descriptions is actually a physical property (shiny=luster, gold=color, shape=crystal form). Physical properties can vary within the same minerals, so caution should be applied. For example, color is a property that is not a very realistic diagnostic tool in many cases. Quartz is a mineral that comes in a variety of colors, as evidenced by Figure 3. Occasionally color can be helpful, as in the case of the mineral olivine. Olivine is said to be “olive green” (a light to dark green) as seen in Figure 4. Make sure you use caution when using color to help identify minerals. We will cover each of the physical properties in detail to help you identify the minerals.

Figure 2 | Describe this mineral.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
Figure 3 | Examples of the different varieties of quartz (jasper, rose quartz, smoky quartz, agate, amethyst, citrine, and petrified wood), demonstrating the difficulty of identifying this mineral.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0

Figure 4 | The mineral olivine is “olive green.”
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
Hardness
Hardness refers to the resistance of a mineral to being scratched by a differ- ent mineral or other material and is a product of the strength of the bonds between the atoms of a mineral. Whatever substance does the scratching is harder; the item scratched is softer. Hardness is based off a scale of 1 to 10 created by a mineralogist named Friedrich Mohs (Figure 5). Mohs’ scale lists ten minerals in order of relative hardness. Each mineral on the scale can scratch a mineral of lower number. Your mineral kit comes with several items of a known hardness. The glass plate has a hardness of 5.5, the iron nail has a hardness of 4, the copper wire has a hardness of 3, and your fingernail has a hardness of 2.5. If you can scratch a mineral, then it would be softer than your fingernail, so therefore its hardness would be <2.5. When trying to scratch a surface, use force, but be cautious with the glass plate. ALWAYS lay the glass plate on a flat surface rather than holding it in your hand in case it breaks. Do not confuse mineral powder with a scratch – use your finger to feel for a groove created by a scratch (mineral powder is left be- hind when a soft mineral scratches a harder surface). Materials of similar hardness have difficulty scratching each other, so that, for example, your fingernail may not be able to always scratch biotite mica, which has a hardness of 2.5.
|
Number |
Mineral |
Hardness of Test Kit Items |
|
1 |
Talc |
(softest mineral) |
|
2 |
Gypsum |
2.5 – Fingernail |
|
3 |
Calcite |
3 – Copper Wire |
|
4 |
Fluorite |
4 – Nail |
|
5 |
Apatite |
5.5 – Glass Plate |
|
6 |
Orthoclase Feldspar |
|
|
7 |
Quartz |
|
|
8 |
Topaz |
|
|
9 |
Corundum |
|
|
10 |
Diamond |
(hardest mineral) |
Figure 5 | Mohs’ Scale of Hardness
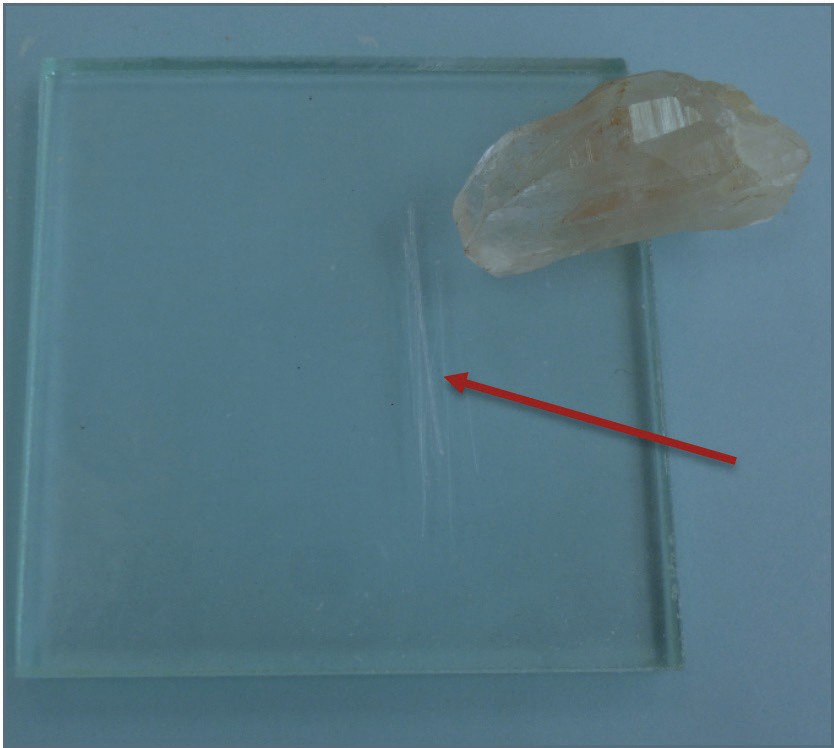
Figure 6 | An example of a scratch made by the mineral quartz on a streak plate. The red arrow is pointing to the scratch. Quartz, therefore, is harder than glass.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0

Figure 7 | An example of a scratch made by a fingernail on the mineral gypsum. The red arrow is pointing to the scratch. Gypsum, therefore, is softer than a fingernail.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
CRYSTAL FORM
This property refers to the geometric shape that a crystal naturally grows into, and is a reflection of the orderly internal arrangement of atoms within the mineral. If minerals have space to grow when they are developing, they will display their crystal form. These ideal growth conditions do not always occur, however, so many minerals do not display their ideal crystal form due to crowded conditions during growth. Examples of crystal form are shown in Figure 9.



Cube Hexagonal Prism Rhombohedron


Octahedron (8 faces) Dodecahedron (12 faces)
Figure 9 | Examples of crystal form
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
CLEAVAGE
As minerals are broken (such as with a rock hammer, for example), some may cleave, or break, along smooth flat planes known as cleavage. These flat surfaces are parallel to directions of weakness within the crystal. All the bonds among the atoms within a mineral may not be of the same strength, so that when a miner- al is broken, it breaks along these zones of weakness. This results in flat cleav- age planes. Minerals with perfect cleavage break along a smooth, flat plane, while those with poor cleavage break in a more irregular fashion. Some minerals do not contain zones of weakness either because all of the bonds are the same strength or the weaker bonds are not aligned within a plane. If this is the case it will not have cleavage, but rather breaks in a random and irregular fashion. Make sure to distinguish cleavage from crystal form. Crystal form occurs as a mineral grows, while cleavage only forms as a mineral breaks. See Figure 7.10 for the main types of cleavage and an example of each.
|
# of Cleavages & Direction |
Cleavage Name |
Example |
|
0 (none) – mineral fractures |
No cleavage planes |
|
|
1 |
Basal cleavage – flat sheets |
|
|
2 – cleavages at or near 90o |
Prismatic cleavage – rectangular cross-sections |
|
|
2 – cleavages not at 90o |
Prismatic cleavage – parallelogram cross-sections |
|
|
3 – cleavages at 90o |
|
|
|
3 – cleavages not at 90o |
|
|
|
4 |
|
|
Figure 10 | Chart with the main types of cleavage, along with picture examples. Red arrows are pointing to the different cleavage planes in each picture.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
A mineral may have one or more cleavage planes. Planes that are parallel are considered the same direction of cleavage and should only count as one. One direction of cleavage is termed basal cleavage. Minerals that display this cleavage will break off in flat sheets. Two directions of cleavage is termed prismatic, while three directions of cleavage at 90o is referred to as cubic. A mineral with four directions of cleavage is termed octahedral. With 2 or more cleavage planes present, it is important to pay attention to the angle of the cleavage planes. To determine the angle of cleavage, look at the intersection of cleavage planes. Commonly, cleavage planes will intersect at 60o, 90o (right angles), or 120o. Be cautious when you see a flat surface on a mineral – not every flat surface is a cleavage plane. Crystal faces can be flat, but remember they form as a mineral grows, while cleavage forms as a mineral breaks. The crystal form of quartz is a hexagonal prism, with nice flat sides. But when quartz is hit with a rock hammer, it breaks in an irregular fashion and does not exhibit cleavage. Also use caution when trying to distinguish the minerals pyroxene and amphibole. Both minerals are black or greenish-black, with similar hardness, making them difficult to tell apart. You must observe the cleavage angles to tell them apart. Cleavage angles in pyroxene are near 90o, so expect it to look boxy and form right angles. Cleavage angles in amphibole are 60o and 120o, so expect a more bladed or pyramid like appearance (Figure 11).
|
Amphibole – cleavage angles at 60o and 120o |
Pyroxene – cleavage angles near 90o |
|
|
|
Figure 11 | Comparison of cleavage angles between amphibole and pyroxene.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
FRACTURE
When minerals do not break along cleavage planes, but rather break irregularly, they are said to fracture. Commonly fracture surfaces are either uneven or conchoidal, a ribbed, smoothly curved surface similar to broken glass (Figure 12).
Figure 12 | This piece of igneous rock called obsidian has been hit with a hammer and is displaying conchoidal fracture.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
LUSTER
Luster refers to the appearance of the reflection of light from a mineral’s surface. It is generally broken into two main types: metallic and non-metallic. Minerals with a metallic luster have the color of a metal, like silver, gold, copper, or brass (Figure 14). While minerals with a metallic luster are often shiny, not all shiny minerals are metallic. Make sure you look for the color of a metal, rather than for just a shine. Minerals with non-metallic luster do not appear like metals. They may be vitreous (glassy), earthy (dull), waxy (similar to a candle’s luster), greasy (oily), or other types (Figure 15).
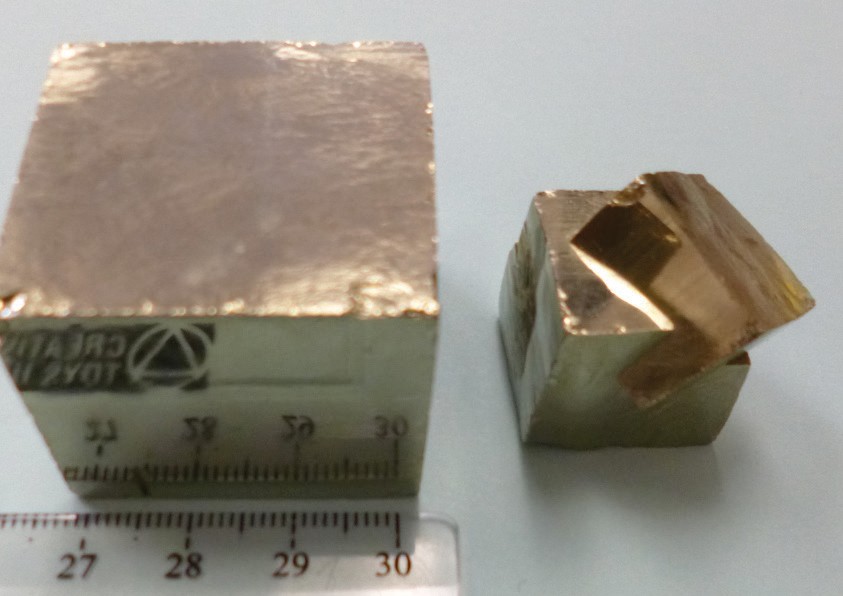
Figure 14 | Examples of the metallic luster of pyrite, also known as “fool’s gold.”
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
|
Vitreous |
|
|
Earthy |
|
|
Waxy |
|
Figure 15 | Examples of different types of non-metallic lusters.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
STREAK
Streak is an easily detectable physical property. It refers to the color left behind on an unglazed piece of porcelain when a mineral is rubbed along its surface. A streak plate is included in your rock and mineral kit to test this property. Often a mineral will have a streak of a different color than the color of the mineral (for example, pyrite has a dark gray streak, Figure 16). Some minerals will have a white streak, which is difficult to see along the white streak plate. If you rub a min- eral along the streak plate and do not see an obvious streak, wipe your finger along the streak plate. A mineral with a white streak will leave a white powder behind that will rub on your finger (Figure 17).

Figure 16 | An example of the dark gray streak left behind when pyrite is rubbed along a streak plate.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0

Figure 17 | An example of the white streak (on finger) left behind when fluorite is rubbed along a streak plate.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
SPECIAL PHYSICAL PROPERTIES
Several minerals have unique proper- ties that aid in their identification. Tenacity refers to the way a mineral resists breakage. If a mineral shatters like glass, it is said to be brittle (like quartz), while minerals that can be hammered are malleable (like copper, Figure 18). Minerals may be elastic, in which they are flexible and bend like a plastic comb, but return to their original shape (like mica, Figure 19). Sectile minerals are soft like wax, and can be separated with a knife (like gypsum).

Figure 18 | Copper, which can be hammered into thin sheets, is malleable.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
Some minerals react when dilute hydrochloric acid is placed on them. Carbonate minerals (minerals that include CO3 in their chemical formula) will effervesce or fizz when acid is applied to them. When you test a mineral with acid, be cautious and use just a drop of the acid. Use your magnifying glass to look closely for bubbles (Figure 20). The acid is very dilute and will not burn your skin or clothing, but wash your hands after use (gloves and goggles are provided). Also make sure that you rinse with water and wipe off the acid from the minerals that you test.
Minerals may be magnetic, and this property is simply tested by seeing if your nail is attracted to a mineral. Magnetite is an example of a magnetic mineral. The mineral halite is simply table salt, so it will taste salty. Graphite is used in pencils, and makes a nice smudge when rubbed along paper. Talc will feel soapy when touched.
Specific gravity is the ratio of a mineral’s weight to the weight of an equal volume of water. A mineral with a specific gravity of 2 would weigh twice as much as water. Most minerals are heavier than water, and the average specific gravity for all minerals is approximately 2.7. Some minerals are quite heavy, such as pyrite with a specific gravity of 4.9-5.2, native copper, with a specific gravity of 8.8-9.0, and native gold at 19.3, which makes panning useful for gold, as the heavy mineral stays behind as you wash material out of the pan.

Figure 19 | Muscovite mica, which bends but returns to its original shape, is elastic.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0

Figure 20 | Note the effervescing acid bubbles at the red arrow on this piece of calcite.
Author: Randa Harris Source: Original Work License: CC BY-SA 3.0
Luster Hardness Cleavage Other Properties Mineral Name
|
Non-Metallic |
> Glass |
Poor Cleavage |
Red-brown, black, silver in color. H=6. St=red-brown |
Hematite |
|
Olive-green in color. H=6. St=white. Commonly granular |
Olivine |
|||
|
Variety of colors. H=7. Conchoidal fracture. Vitreous luster. |
Quartz |
|||
|
Clearly Shows Cleavage |
Black to greenish black in color. H=6. C=2 planes at ~60o and 120o. Elongated crystals. |
Amphibole |
||
|
Tan-pink, white, green in color. H=6. C=2 planes at 90o. |
Microcline |
|||
|
Black to greenish black in color. H=6. C=2 planes at ~90o. Short stub- by crystals. |
Pyroxene |
|||
|
< Glass |
Poor Cleavage |
Dark gray to black in color. H=1. Greasy feel – will smudge fingers. |
Graphite |
|
|
Yellow in color. H=1.5-2.5. St=white to yellow. |
Sulfur |
|||
|
White to green in color. H=1. Soapy feel. |
Talc |
|||
|
Clearly Shows Cleavage |
Brown to black in color. H=2.5. C=1 perfect. Breaks into thin sheets that are elastic. |
Biotite Mica |
||
|
White to transparent in color. H=3. C=3 rhombohedral. Strong efferves- cence in acid. |
Calcite |
|||
|
Transparent, yellow, purple, green in color. H=4. C=4 – octahedral. |
Fluorite |
|||
|
Transparent to white in color. H=2. C=3, though 2 directions may be difficult to see. |
Gypsum |
|||
|
White to transparent in color. H=2.5. C=3, cubic. Tastes salty. |
Halite |
|||
|
Transparent, light brown, to yellow in color. H=2.5. C=1 perfect. Breaks into thin sheets that are elastic. |
Muscovite Mica |
|||
|
Metallic |
> Glass |
Poor Cleavage |
Black in color. H=6. St=black. Strongly magnetic. |
Magnetite |
|
Brass-yellow in color. H=6.5. St=dark gray. |
Pyrite |
|||
|
<Glass |
Poor Cleavage |
Copper-red in color. Tarnishes to green or black in air. H=2.5-3. St=copper-red. |
Copper |
|
Figure 22 | Mineral Identification Chart
















Feedback/Errata