Part Four: Sustainability and the Earth’s Future
Lab 12 Reading: Resources and Sustainability
OBJECTIVES
- Understand how humans use resources.
- Be able to relate the geologic environments in which we find different rocks and minerals to how we find resources to mine.
INTRODUCTION
Do you like all that stuff you own? Take a look around the room. Did you ever stop to think where all the materials that were used to create the desk, chairs, walls, or floor came from? Take a look at your phone or your computer. Did you ever wonder what all those little components are made of? Everything you see around you required the use of some mineral resource. Even that apple you ate for a snack required mineral resources: a truck likely drove it from the orchard to you, and that truck was made out of some combination of metals which had to be mined from a mineral resource. So what is a resource? A resource is any earth material that humans use and find useful.
Natural resources like water, soil (land), and air are considered essential resources. These are necessary for human life. Other resources like mineral resources and energy resources (fossil fuels, geothermal energy, and solar energy) are also important for human survival. Resources are also defined as renewable (or infinite) and nonrenewable (finite). A renewable resource is replenished or recreated within a human lifetime. For instance, a tree is a renewable source of energy: a tree can be regrown in 35-50 years, which is within the timeframe of a human life. However, coal resources are not renewed within a human lifetime. These resources took at least hundreds of thousands of years to form. Once these are used, they will not be replenished within a human lifetime.
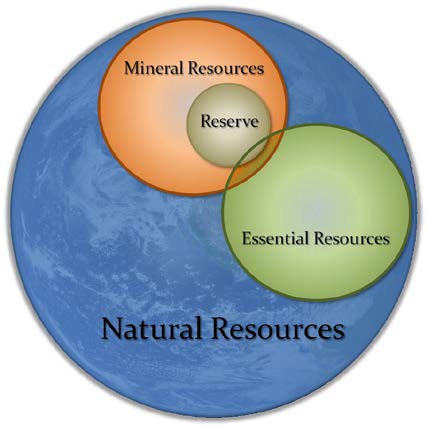
FIGURE 1 Diagram showing the relationship between natural resources, essential resources, and mineral resources, including mineral reserves.
Mineral and rock resources concentrate in areas due to different processes. By matching the process with the rock or mineral we want, we can then search for the geologic settings which create these rocks or minerals. See the table below.
|
Geologic Process |
Mineral or Rock Resource |
|
Igneous |
Diamonds – South Africa |
|
|
Chromite – Stillwater, Montana |
|
|
Magnetite – Andirondack Mountains, New York |
|
|
Beryl and lithium – Black Hills, South Dakota |
|
Copper – Butte, Montana |
|
|
Metamorphic |
Lead and Silver – Leadville, Colorado |
|
|
Asbestos – Quebec, Canada |
|
Sedimentary |
Potassium – Carlsbad, New Mexico |
|
|
Halite (salt) – Oklahoma and Kansas |
|
|
Gold – Sierra Nevada foothills, California |
|
|
Sand and Gravel – Northern and Southern Indiana |
|
|
Manganese oxide nodules – central and southern Pacific Ocean |
|
Coal – Southern Indiana |
|
|
Biological |
Phosphorus – Florida |
|
Weathering |
Bauxite – Arkansas Copper – Utah |
TABLE 1 Relationship between geologic processes and different mineral resources. (Modified from Edward A. Keller. 2011. Introduction to Environmental Geology, 5th ed. Upper Saddle River, NJ: Prentice Hall.)

FIGURE 2 A mine is an area of the Earth’s surface (or just below) that is designated for extracting mineral resources. Mines can look an open pit, Figures 2A and 2B. Picture A shows a gold mine in Nevada. (Image Credit: Nevada Bureau of Mines) Picture B is an active coal mine near Evansville, Indiana. (Image Credit: Tom Barrows, 2009) Picture C shows an underground room and pillar salt mine. (Image Credit: Kansas Geological Survey, 1978)
PART 12A: IGNEOUS PROCESSES
You may recall that igneous rocks are “new rock” formed from cooled magma either above or below the Earth’s surface. The way that minerals form in a magma chamber can concentrate different mineral resources.
Scientists have observed that minerals form at different temperatures, for instance, olivine crystals form at the hottest temperatures, 1000 – 1200 °C and quartz crystals form when the magma chamber has cooled to around 720 °C. This progression of mineral crystalization with cooling temperatures is called Bowen’s Reaction Series. When crystals form in a magma chamber, they are often more dense and heavier than the surrounding molten rock. This causes these minerals to settle to the bottom of the magma chamber through a process called crystal settling. As the magma chamber cools, different new minerals form and settle to the bottom, creating layers or veins of minerals. Chromite (FeCr2O4) is an important mineral ore which is found in these old, layered magma chambers. This is an important resource for anyone who likes to look at him or herself in a mirror or cook with stainless steel cookware: it is a major ore for chromium which is used to make important metal alloys and shiny metal coatings (plating). We find crystal settling in old intrusive igneous bodies.
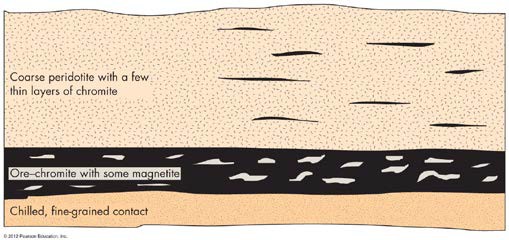
FIGURE 3 How chromite layers might form. The chromite crystalizes early, and the heavy crystals sink to the bottom and accumulate in layers. (From Foster, R.J. 1983. General Geology, 4th ed. Columbus, OH: Charles E. Merrill)
Diamonds or the mineral kimberlite is also formed from an igneous process. Diamonds are found in old kimberlite pipes, huge cone shaped spikes of igneous rocks that have a wide diameter at the top and taper down a couple hundred kilometers below the Earth’s surface. Within these pipes, carbon is exposed to high temperatures and pressures over very long periods of time. The kimberlite pipes in South Africa are billions of years old. Younger kimberlite pipes are found in the continental United States in Arkansas. You can go hunting for diamonds in Arkansas’s Crater of Diamonds State Park. Magma chambers under old continental crust are areas where these kimberlite pipes have formed.
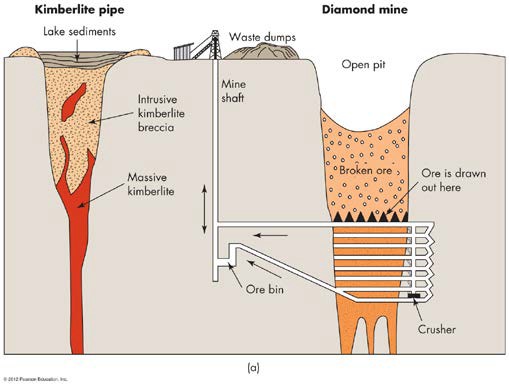
FIGURE 4 An idealized diagram showing a typical South African diamond pipe and mine. Diamonds are scattered throughout the cylindrical cone body of igneous rock, which is kimberlite. (From Kesler, S.E. 1994. Mineral Resources, Economics and the Environment. New York: Macmillan)

FIGURE 5 Kimberlite with a diamond. (Pearson Education, Inc. 2012)
When a magma chamber happens to heat up rock with water, that water becomes a chemically active fluid, able to dissolve, oxidize, and hydrolyze different mineral components. This liquid is highly concentrated with minerals that are the last to crystalize based on Bowens Reaction Series, often feldspars and quartz.
When the water retreats, the minerals recrystallize in the cracks within the rocks. This creates veins of hydrothermal (hot water) deposits. This process also concentrates gold, silver, copper, mercury, zinc, and many other metals. This igneous process occurs with contact metamorphism and is responsible for concentrating copper in areas near Butte, Montana and areas of Northern Chile. See Figure 6.
PART 12B: METAMORPHIC PROCESSES
Both contact metamorphism and regional metamorphism can concentrate different minerals. Both of these can result in the formation of chemically active fluids as well as change existing sedimentary rocks into more usable metamorphic rocks like marble.
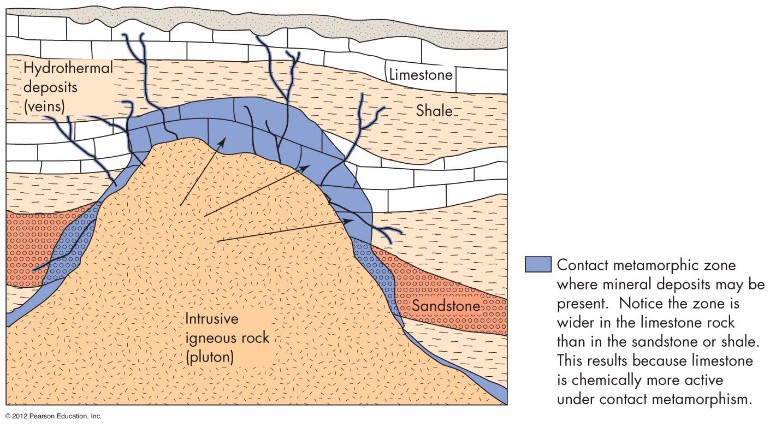
FIGURE 6 Hydrothermal and contact metamorphic ore deposits. The dark blue veins show where recrystallized minerals have formed in cracks between the sedimentary rocks. The lighter blue shows where contact metamorphism has changed the rocks, concentrating different mineral resources like marble from the metamorphism of the limestone. (From Edward A. Keller. 2011. Introduction to Environmental Geology, 5th ed. Upper Saddle River, NJ: Prentice Hall)
PART 12C: SEDIMENTARY PROCESSES
Sand and gravel deposits are formed from depositional processes such as glacial deposits or stream deposits. As we learned when we covered sedimentary rocks, different depositional processes sort different sized sediments.
If you have ever watched a movie or seen a picture of someone panning for gold, you have seen someone taking advantage of a specific stream sorting process which creates placer deposits. Placer deposits are formed along bends in streams (meanders). As a stream is transporting its suspended and bed load, heavier minerals and rocks are deposited in the middle, toward the inside of the bend. This is because water moves faster on the outside of the bend, just like a race car driver has to go faster on the outside of a turn to keep pace with (or pass) a car on the inside of the turn. Because the inside bend of a stream has slower moving water, larger heavier minerals and rocks are deposited in these areas. Over thousands of years, this process can concentrate large amounts of heavier minerals like gold. See Figure 7.
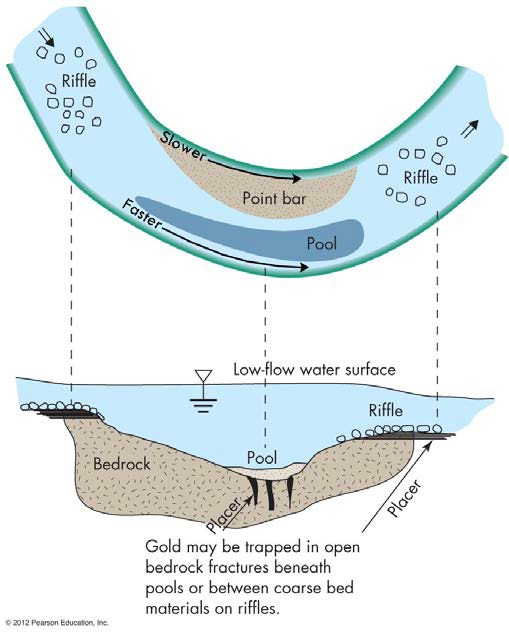
FIGURE 7 How placer deposits of gold might form. The heavier gold is deposited in the slower moving parts of the stream. (From Edward A. Keller. 2011. Introduction to Environmental Geology, 5th ed. Upper Saddle River, NJ: Prentice Hall)
When large shallow seas evaporate, they can create layers of chemical sedimentary rocks called evaporites. These evaporite deposits then form mineral resources like salt (NaCl, halite), gypsum, and anhydrite. Some evaporite deposits like halite become mobile over geologic time. Because the salt is less dense than the surrounding rock, it rises, forming large salt domes. These are found in old intraplate settings. See Figure 8.
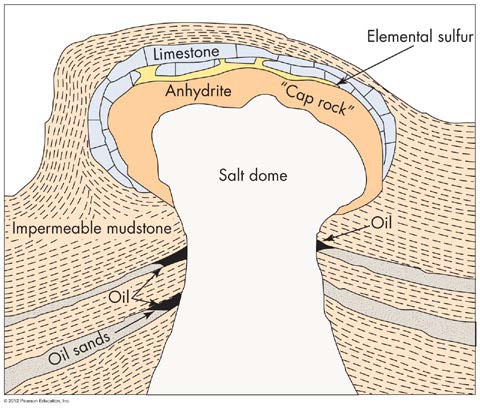
FIGURE 8 A salt dome rising through sedimentary rock layers. (From Edward A. Keller. 2011. Introduction to Environmental Geology, 5th ed. Upper Saddle River, NJ: Prentice Hall)
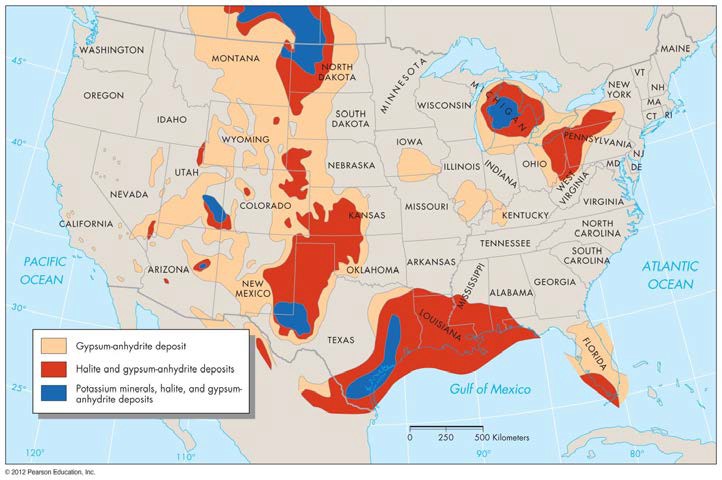
FIGURE 9 Location of evaporite deposits in the United States. (From Brobst, D.A., and Pratt, W.P., eds. 1973. U.S. Geological Survey Professional Paper 820)
Sedimentary processes are also responsible for concentrating fossil fuel resources like coal and oil (petroleum). Coal is formed from the sedimentation of bioclastic material like plants in oxygen poor environments (old swamps). Therefore, we can find coal in areas where sedimentary rocks formed in old swamps. See Figure 10.
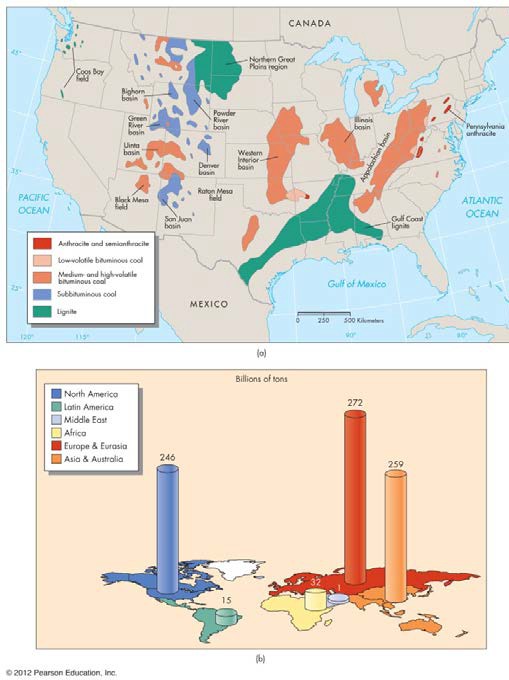
FIGURE 10 Distribution of coal reserves in the United States (above) and the world (below). (From Edward A. Keller. 2011. Introduction to Environmental Geology, 5th ed. Upper Saddle River, NJ: Prentice Hall)
Oil is formed from similar processes; however, it forms in old basins, some from as far back as the Paleozoic Era – but most are Cenozoic age. These depositional basins continue to bury organic-rich sediments (called source rock). As these are buried to depths of 1 – 3 km, they are exposed to increased temperatures which force a chemical change in the organic debris, changing it into thermogenic oil and natural gas. These need to be trapped by overlying impervious rocks; otherwise, they would bubble to the surface and be lost. Therefore, in order for oil to be concentrated, there needs to be a “trap” rock, called a cap rock, which allows the oil to rise and accumulate, but not be lost to the surface. We often find oil trapped along the sides of salt domes, under anticlines, faults, or under unconformities. In the case of oil, it’s not enough to just have sedimentary rock, but we also need some other geologic process like an intruding salt dome, compressional tectonic activity, or an unconformity to concentrate the oil (and often, natural gas). See Figure 11.
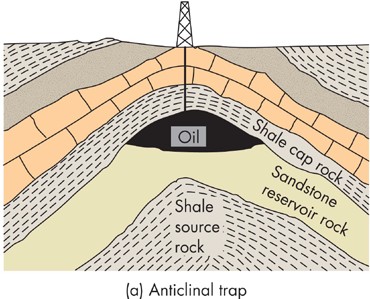
FIGURE 11 Structural oil traps. From Edward A. Keller. 2011. Introduction to Environmental Geology, 5th ed. Upper Saddle River, NJ: Prentice Hall)
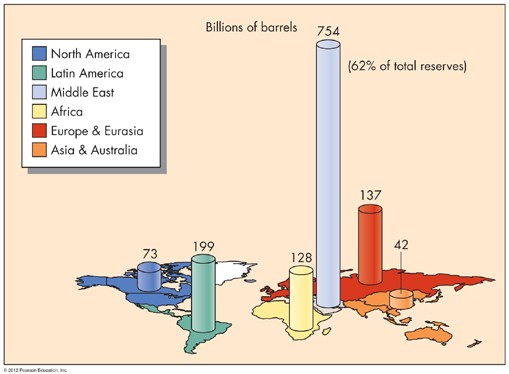
FIGURE 12 Distribution of oil reserves around the world. (From Edward A. Keller. 2011. Introduction to Environmental Geology, 5th ed. Upper Saddle River, NJ: Prentice Hall)
PART 12D: BIOLOGICAL PROCESSES
Different biological processes also help to concentrate mineral resources. For instance, we learned that sedimentary rocks like chalk and coquina limestones are made from the sedimentation of organisms. These organisms, in their lifetime, concentrated calcium carbonate in their bodies. Organisms also concentrate large amounts of phosphates. Ancient reefs created large deposits of fossiliferous limestone (calcarenite).
This is an incredibly important resource for agriculture as it is where we obtain phosphorus for fertilizers. The major deposits of calcarenite in the United States are found in Florida. At current rates of usage, the United States only has about 15 – 20 years left of this resource. The next largest reserve is in Morocco with China having the largest supply. See Figure 13.

FIGURE 13 Open-pit phosphate mine in Florida. (From Edward A. Keller. 2011. Introduction to Environmental Geology, 5th ed. Upper Saddle River, NJ: Prentice Hall)
PART 12E: WEATHERING PROCESSES
Residual ore deposits are formed from differential weathering of minerals in soils. As water moves down through soil layers, it dissolves away easily soluble minerals like carbonates. Over time, this leaves behind less easily soluble minerals like oxides. Oxides are metal bearing minerals. Therefore, these metal bearing minerals become concentrated in the upper soil layers. These oxide-rich soils are called laterites. Aluminum oxide or bauxite is found this way. (This how the aluminum for foil and soda cans is mined.) Instead of creating an open-pit mine, bauxite is mined by scraping off the upper layers of sediments in areas where laterite soils are found. Nickel and cobalt are also found and mined this way. See Figure 14.

FIGURE 14 Surface bauxite mine in Australia. (Image credit: Luis Castaneda/Getty Images)
PART 12F: OTHER PLACES WE FIND MINERALS
In recent years with increased ocean exploration, ocean sediments have been found which also have high concentrations of usable minerals. Sulfide deposits around hydrothermal vents (black smokers) are helping geologists better understand sulfide deposits, allowing them to better find land deposits of sulfide minerals.
Manganese oxide nodules and cobalt-enriched manganese crusts are being mined for manganese- oxides, iron-oxides, nickel, platinum, copper, and molybdenum.
PART 12G: RESERVES VERSUS RESOURCES
It’s important to understand not only how and where mineral and rock resources are found, but to also understand the processes which concentrate them. If minerals and rocks are not found in high concentrations, it is not profitable to mine them.
When a resource is found in concentrations which are profitable to extract, the resource is now called a reserve. Companies are only going to mine minerals if they are profitable. See Table 2 below. This shows the approximate profitable concentration factor for selected metals. If a metal is not found in at least that concentration, then the metal would not be profitable to mine (by current market values).
In recent years, metal prices have increased. As prices increased, it becomes more profitable to mine once unprofitable sources of minerals and rocks. For instance, gold is becoming scarcer in the Earth’s crust. It is estimated that there are only about two Olympic sized swimming pools of gold in all the Earth’s upper crust and most of it has been extracted. It is now profitable for companies to “mine” gold from the human population, which is why you see advertisements and store fronts for “gold for cash” businesses. With current gold prices exceeding $1,500 per ounce and increasing market values for transition metals like Niobium and Tatalum (found in the mineral Coltan, Columbite-Tantalite), it is also becoming profitable for recycling companies like cash for gold businesses and gazelle.com to buy back gold and old electronics to remove and scrape out the minute amounts of gold and other precious metals to be reused in new electronics.
This increase in market values is also driving the United States to open up new mines, especially, mines that can profitably extract rare earth metals (which are not technically rare, but are called rare based on their designation in the periodic table). These metals such as Scandium, Lanthanum, Praseodymium, Erbium, and Lutetium are used in high performance, compact electronics (found in cars, cell phones, tablets, and computers) and medical imaging devices.

FIGURE 15 Historically high prices of gold now make it profitable for companies to “mine” gold from the human population.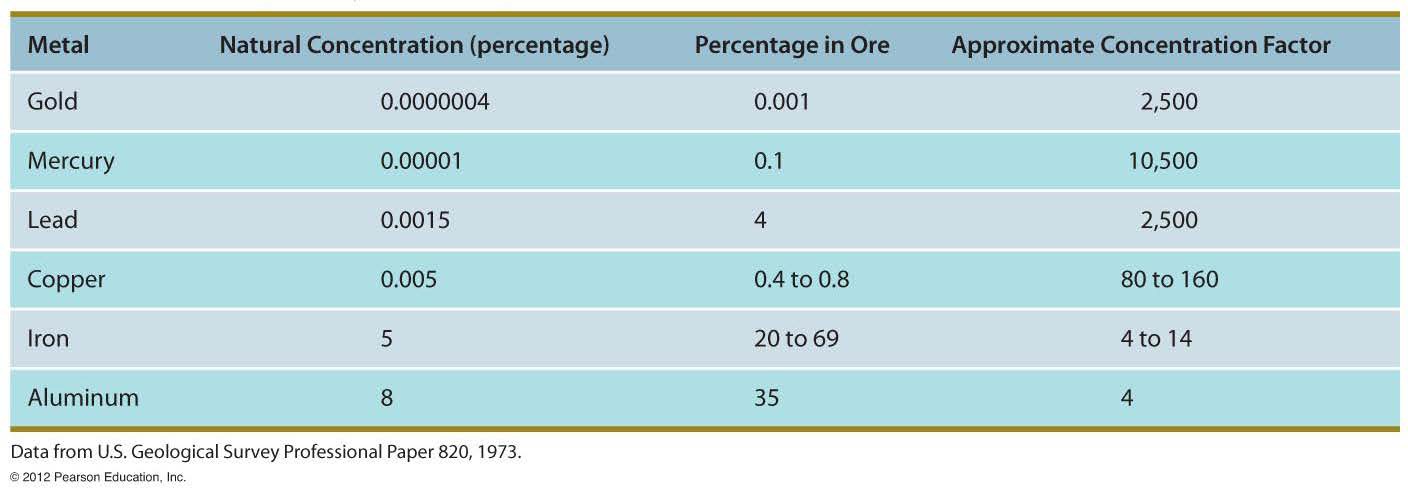
TABLE 2 Table of approximate concentration factors of selected metals. Metal resources must be found at these concentration factors before mining is economically feasible. (From Edward A. Keller. 2011. Introduction to Environmental Geology, 5th ed. Upper Saddle River, NJ: Prentice Hall)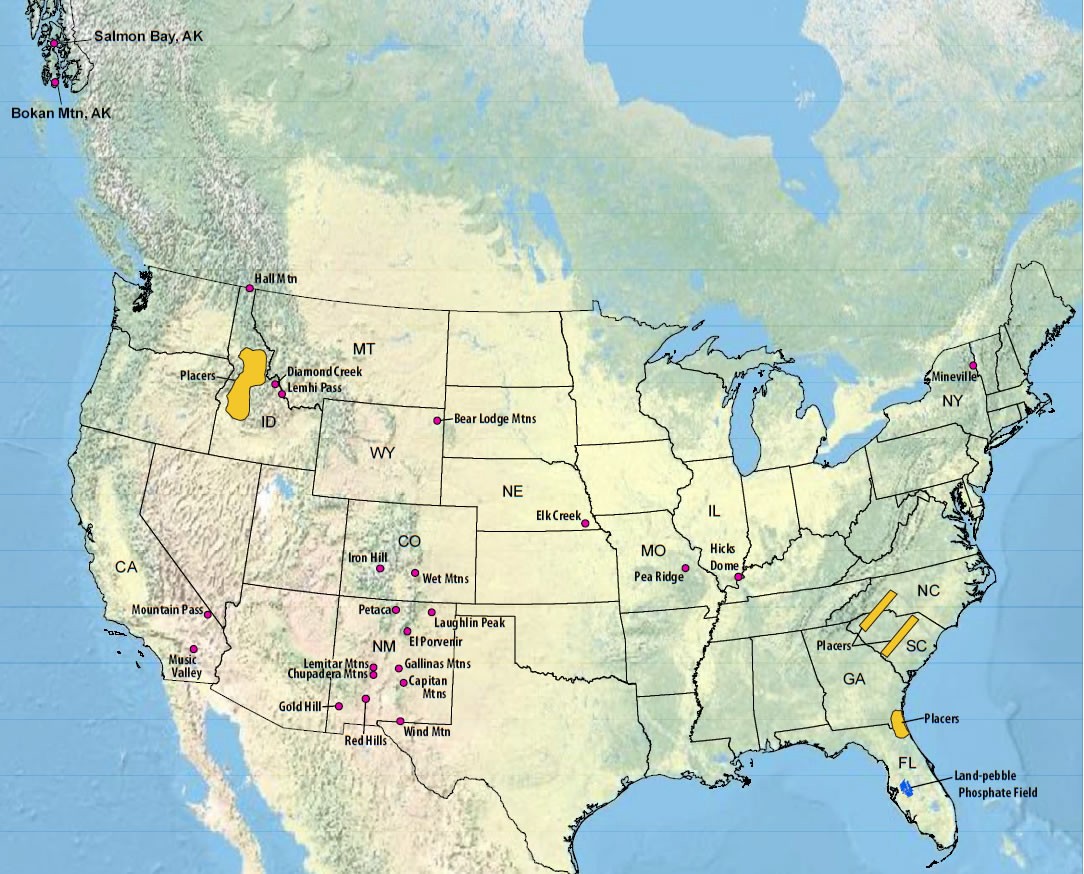
FIGURE 16 Location of rare earth metal mines in the United States. (Map credit: geology.com)
PART 12H: MINERALS AROUND YOU
According to the Minerals Education Coalition, the average American uses 38,212 pounds of mineral and rock resources each year. (See Figure 17.)

Figure 17 List of common mineral and rock resources and amounts used by the average American. (Figure Credit: Minerals Education Coalition)


Feedback/Errata