4 Conductivity of Aqueous Solutions
Purpose
To investigate and explain factors affecting the solubility of different aqueous solutions.
Learning Outcomes
After completing this experiment, you should be able to
- Describe and explain the difference in conductivity between strong electrolytes, weak electrolytes, and non-electrolytes.
- Describe and explain the relationship between the concentration of ions and the conductivity of solutions.
Textbook Reference
Tro, Chemistry – A Molecular Approach (5th Ed), Ch. 5.4.
Introduction
The Behavior of Compounds When Dissolved in Water
When dissolved in water, ionic and molecular compounds behave differently:[1]
Ionic Compounds
When ionic compounds dissolve in water, they would dissociate into their constituent ions when they dissolve in water.
Example
When potassium chloride dissolves in water, the crystal structure breaks apart such that the potassium and chloride ions dissolve separately in water.
(1) ![]()
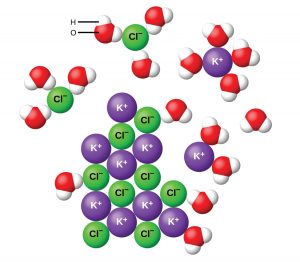
Note that polyatomic ions will remain as discrete units; when dissociation occurs, covalent bonds will not be broken. You will also need to pay attention to the number of ions present.
Example
When sodium sulfate dissociates in water, the sulfate ions do not dissociate further. This is what we would expect to find.
(2) ![]()
Note that:
- Since there are two sodium ions in
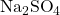 , there will be two of them in solution per formula unit of sodium sulfate.
, there will be two of them in solution per formula unit of sodium sulfate. - The sulfate ion (
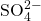 ), does not dissociate further as it is held together by covalent bonds. Therefore, you expect to see sulfate ions present together. It is therefore critical that you memorize your polyatomic ions.
), does not dissociate further as it is held together by covalent bonds. Therefore, you expect to see sulfate ions present together. It is therefore critical that you memorize your polyatomic ions.
Molecular Compounds
As these are held together by covalent bonds which will not break when dissolved in water, these do not dissociate when dissolved in water.
Example
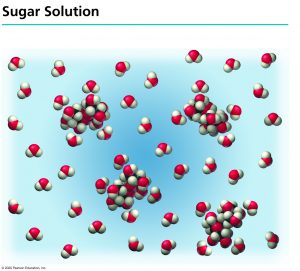
When sucrose (![]() ) is dissolved in water, it does not break down. The sucrose molecule will remain intact and no ions will be formed from it.
) is dissolved in water, it does not break down. The sucrose molecule will remain intact and no ions will be formed from it.
We have to be more cautious about acids and bases, however. Acids and bases ionize and form charged particles in solution. Of these, strong acids such as HCl would ionize completely (i.e. all of the molecules react with water to form the constituent ions):[2]
(3) ![]()
On the other hand, most acids and bases (weak acids and bases) do not ionize completely; only a small proportion of ions are ionized, as illustrated for acetic acid below. As a result, there is a dynamic equilibrium.[3]
Conductivity of Aqueous Solutions
It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. We can also distinguish between these within the realm of aqueous solutions – solutions where the solvent is water.
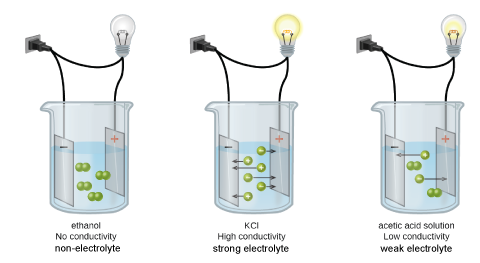
Types of Aqueous Solutions
Non-Electrolytes
Non-electrolytes do not conduct electricity when dissolved in water. These compounds are \emph{molecular} and are not acids or bases and hence will not dissociate in water.
Electrolytes
These solutions contain ions, and since there are freestanding charged particles in the solution, they conduct electricity to varying extents. Since the mobility of different types of ions in solution vary significantly, so will the conductivity. Regardless, two different factors that we understand will affect the conductivity significantly:
-
The greater the concentration of ions present in a solution, the greater its conductivity.
- The type of electrolyte. We can classify electrolytes as weak or strong electrolytes:
- Weak electrolytes do not dissociate/ionize much, they tend to have significantly lower ion concentrations and therefore they do not conduct as much as strong electrolytes.
- Strong electrolytes dissociate/ionize completely; none of it would not dissociate and they tend to conduct well.
Salts are generally strong electrolytes. For acids and bases, your strong acids and bases are strong electrolytes while your weak acids and bases are weak electrolytes.
- There are six main strong acids: HCl, HBr, HI (not HF), H2SO4 (first proton only), HClO4, HNO3. All other acids are weak acids (for the purpose of this class).
- The main strong bases we are concerned with are Groups I and II hydroxides (and a few others that we don’t worry about too much in this class). Other bases are typically weak.
Measurement of Conductivity[4]
In the second part of this experiment, you will measure the conductivity of a solution. The conductivity of a solution can be measured using a conductivity meter (which has an electrode). This is measured in units of Siemens per meter (![]() ).
).
As far as this course is concerned, you are not required to understand the physics associated with conductivity; you just need to be able to recognize that these are values of conductivity
Experimental Procedures
There are two parts to this experiment. In the first part of the experiment, you will use the PhET Sugar and Salt Solution simulation to visualize how salts dissociate. In the second part of the experiment, you will use Gary Bertrand’s Conductivity simulation to examine how different solutions have different conductivities.
Electrolytes versus Non-Electrolytes
Open the PhET Sugar and Salts solution simulation and open the Macro tab.
- Put the conductivity probe into the solution. What happens to the light bulb?
- Add salt from the shaker and see what happens to the light bulb. What happens when you add even more salt?
- Remove all the salt in the container and repeat (2) with sugar instead of salt.
Now, open the Micro tab. Put some of each substance in turn into the container and draw what you see. Take a screenshot of the container with salt and with sugar.
Conductivity of Different Solutions
Open the Gary Bertrand’s Conductivity simulation. The way this works is that you select:
- The cation
- The anion
- The concentration
and then the beaker on the right will magically contain that solution. To measure the conductivity of that solution (in μS), press the right hand side of the conductivity meter (purple bar).
Conductivity of Water
Measure the conductivity of pure water.
Comparing the Conductivity of Different Electrolytes
Measure the conductivity of 0.0050 M solutions of each of the following:
- HCl
- HNO3
- KOH
- KCl
- KNO3
- Ca(NO3)2
- NH4OH (=NH3)
- HC2H3O2
Comparing the Conductivities as a Function of Concentration
Measure the conductivity of HCl from 0.0010 to 0.0050 M at 0.0010 M increments.
- This will be explained later in Tro, Ch. 10.4. ↵
- This is a simplification, which we will break down next semester in CHEM-C 106 (Principles of Chemistry II). ↵
- We will explore this much more next semester in CHEM-C 106. ↵
- You may be more familiar with the concept of resistivity, which is the inverse of conductivity. ↵
