8 Chemical Equilibria: Le Châtelier’s Principle and its Application
Purpose
To evaluate the effect of temperature and addition of reactants on the position of equilibrium in chemical reactions in equilibrium.
Learning Outcome
- Apply le Châtelier’s Principle to predict the effect of a change in (a) concentration and (b) temperature on the position of equilibrium.
- Determine the sign of the heat of reaction based on the equilibrium shift.
Textbook Reference
- Tro, Chemistry: Structure and Properties, 2nd Ed., Ch. 15.9.
- Tro, Chemistry: A Molecular Approach, 5th Ed., Ch. 16.9.
Theoretical Background
Chemical Equilibrium: A Dynamic Process
![As timew goes on, the % [A] decreases and [B] increases](https://upload.wikimedia.org/wikipedia/commons/3/33/Dynamic_equilibrium.png)
In CHEM-C 105/125, the focus (in terms of chemical reactions) was on reactions where the reaction goes to completion. In this case, at the end of the reaction, we expect all of the limiting reactant to react to form product, with no limiting reactant left; the reverse reaction will not occur. However, in many cases, the reaction can occur in both directions. Consider the hypothetical reaction:
![]()
If I start with pure \ce{A}, then clearly no reverse reaction occurs; only the forward reaction occurs, and A is converted to B. However, as this proceeds, as ![]() decreases, the rate of the forward reaction decreases. Conversely, the reverse reaction (
decreases, the rate of the forward reaction decreases. Conversely, the reverse reaction (![]() ) occurs with an increasing rate as
) occurs with an increasing rate as ![]() increases.
increases.
Eventually, as the rates converge, the forward reaction rate will equal the reverse reaction rate. At that point, the macroscopic concentrations of reactants and products will remain constant. However, it is a dynamic equilibrium; while the concentrations of reactants and products remain constant, the forward and reverse reactions continue to proceed at equal rates.
Le Châtelier’s Principle
We can use Le Châtelier’s Principle to predict the direction to which a system will shift when a change is made to a system at equilibrium. It states that
If a stress is applied to a reacting system in equilibrium, the position of equilibrium shifts such that the effects of the stress is negated.
That is to say, if you add or remove something from the system, the reacting mixture shifts its position to “undo” whatever change you have made.
Effect of Changing Concentration on Position of Equilibrium
When the concentration of a species (reactant or product) is decreased, the system shifts its position to increase the concentration of the species whose concentration was decreased. Conversely, when a species is added, the reaction position shifts to the other side as that specific species is reacted.
We can account for this by calculating the reaction quotient for this reaction and showing the direction to which the equilibrium will shift. The equilibrium constant will remain constant in this case. Using an ICE/equilibrium table, you can calculate the final, resultant concentrations at equilibrium.
Effect of Changing Temperature on the Position of Equilibrium
To think about this, we can use the idea that increasing the temperature is, in a conceptual sense, “adding” heat; decreasing the temperature would be “removing” heat. In turn, depending on whether the forward reaction is endothermic or exothermic, we can determine whether heat is a reactant or product:
- Endothermic reactions (
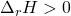 ) would have energy transferred as heat from the surroundings into the system, and therefore heat would be a reactant (has to be put into the system).
) would have energy transferred as heat from the surroundings into the system, and therefore heat would be a reactant (has to be put into the system). - Exothermic reactions (
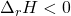 ) would have heat transferred from the system into the surroundings. Therefore, heat is a product of this reaction.
) would have heat transferred from the system into the surroundings. Therefore, heat is a product of this reaction.
The easiest way to look at this would be to write down heat explicitly into the equation as a reactant or product, and analyze this similarly to adding/removing a particular reactant/product from a reacting mixture.
Example
The reaction
![]()
is endothermic. Therefore, heat can be written in to the equation on the reactants side:
![]()
When the temperature increases, you are adding heat to the system – which can be treated as a reactant. Therefore, the equilibrium shifts to the right – more \ce{N2O4} is produced from \ce{NO2}, as illustrated below:

Note that temperature changes will alter the value of the equilibrium. In the experiment Thermodynamics of the Solvation of Salts, you will study how the equilibrium constant changes as a function of temperature.
The Tetracolbaltate(II) Reaction System

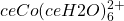 ) on the left and in excess chloride (with
) on the left and in excess chloride (with 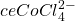 ) on the right. Credit: Leiem
) on the right. Credit: LeiemIn the presence of chloride ions, cobalt (II) undergoes the following reaction to form tetrachlorocobaltate (II):
![]()
Chloride ions, however, can be removed from the solution by adding silver nitrate from the solution. When silver ions are added to the solution, the chloride ions are precipitated out and removed from the solution.
![]()
As the two compounds have different colors as shown on the right, we will be able to visualize the position of equilibrium for this reaction. This can be done using the virtual experiment setup from ChemCollective.
Procedures
Effect of Concentration on Equilibrium
Open the Cobalt Chloride and LeChatlier’s Principle simulation on ChemCollective. You are provided with solutions of 1 M cobalt(II) chloride, 12 M hydrochloric acid, and 6 M silver nitrate.
When you click on a solution, you will be provided with the concentrations of the different species in that given container. This gives conveniently a way for you to look at the concentrations of different solutions:
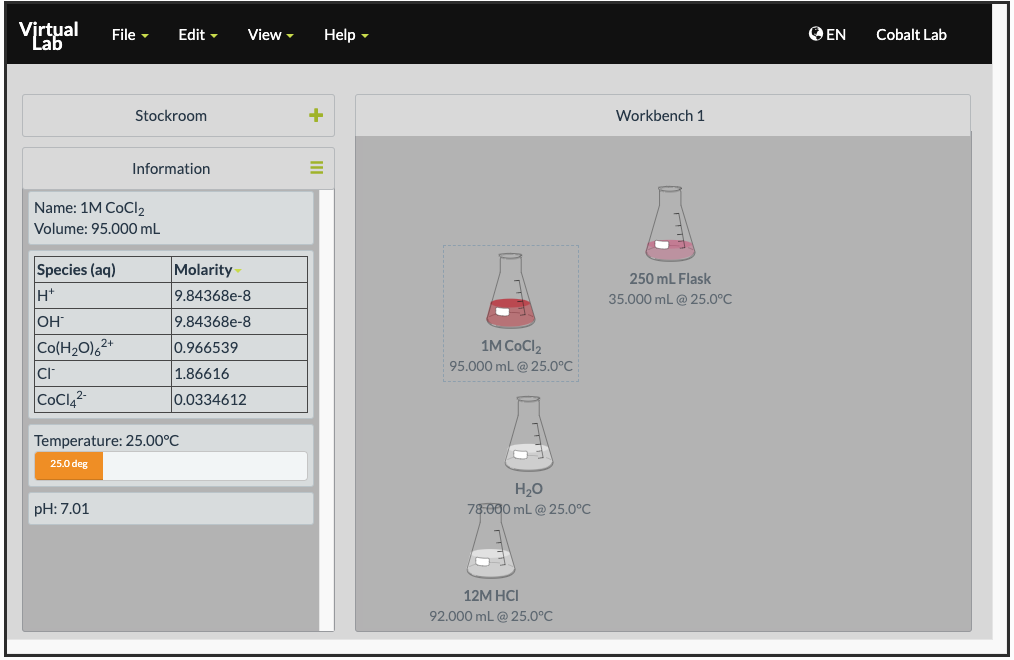
Effect of Adding Chloride Ions
- Collect a 250 mL beaker and pour 5 mL of the 1 M CoCl2 solution. Record the color of the solution and the concentrations of
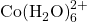 and
and 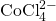 .
. - Add 2 mL of 12 M HCl(aq) into the beaker. Record the color of the solution and the concentrations of
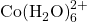 ,
, 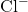 and
and 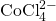 . What happens to the temperature of the solution?
. What happens to the temperature of the solution? - Repeat step 2 seven more times.
Effect of Removing Chloride Ions
- Starting from the beaker you prepared above, add 4 mL of 6 M AgCl to the beaker. Record the color of the solution and the concentrations of
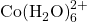 ,
, 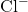 and
and 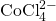 .
. - Repeat step 1 seven more times.
Effect of Temperature on the Equilibrium
Given what you have done so far, design and implement an experiment to demonstrate both qualitatively and quantitatively how the position of equilibrium shifts with temperature.
