10 Thermodynamics of the Solvation of Salts
Yu Kay Law
Purpose
To determine ![]() , and
, and ![]() for the solvation of different salts.
for the solvation of different salts.
Expected Learning Outcomes
- Define and measure the solubility product constant for a salt at different temperatures.
- Determine thermodynamic parameters associated with chemical equilibria.
Textbook Reference
Tro, Chemistry – Structures and Properties, 2nd Ed, Ch. 17.5 (Ksp) and 18.9 (Gibbs energy and chemical equilibria).
Introduction
Describing Solubility Using the Language of Chemical Equilibria
In the chapter on solubility, we described the solubility in terms of the mass of solute dissolved per 100 g of solvent. Fairly straightforwardly, we can also quantify the solubility by the molarity of a saturated solution: the molar solubility.
For an ionic compound, when it dissolves it would dissociate into its constituent ions. For a slightly soluble compound such as potassium dichromate (K2Cr2O7), for example,
(1) ![]()
where we treat the process as an equilibrium process. With the solvation process being treated as an equilibrium, we can write its equilibrium constant as
(2) ![]()
where we call the equilibrium constant for the dissociation of an ionic compound in solution the solubility-product constant Ksp. Given the molar solubility, we can find the Ksp value using an ICE table as we did in Ch. 15. This is further explained in the following video:
https://iu.mediaspace.kaltura.com/id/1_31k4k1ff?width=400&height=285&playerId=26683571
Gibbs Free Energy and the Equilibrium Constant
The Gibbs Free Energy
We know that, for a spontaneous process under constant pressure and temperature, the Gibbs energy of a process, defined as
(3) ![]()
would decrease. Furthermore, at equilibrium, it can be shown that ![]() . Furthermore, it can be shown that the standard Gibbs free energy is related to the equilibrium constant by
. Furthermore, it can be shown that the standard Gibbs free energy is related to the equilibrium constant by
(4) ![]()
Assuming that ![]() and
and ![]() are temperature-independent, we can relate the equations above to yield the equation
are temperature-independent, we can relate the equations above to yield the equation
(5) ![]()
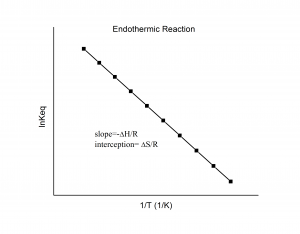
where ![]() is the ideal gas constant. From this equation, you can show that if you plot ln K (along the y-axis) against 1/T (along the x-axis, with temperature in Kelvins) you should yield a straight line with the slope being
is the ideal gas constant. From this equation, you can show that if you plot ln K (along the y-axis) against 1/T (along the x-axis, with temperature in Kelvins) you should yield a straight line with the slope being ![]() and the y-intercept being
and the y-intercept being ![]() .
.
In this experiment, you will measure the ![]() for two different salts at different temperatures. You will then find
for two different salts at different temperatures. You will then find ![]() and
and ![]() of the solvation process.
of the solvation process.
Procedure
You will perform this experiment using the ChemCollective Temperature and the Solubility of Salts virtual lab – it’s unrealistic but it would illustrate the chemical concepts.
Each student will study the solubility of three solids: cerium sulfate (Ce2(SO4)3; there is a typo in the name on the Virtual Lab) and two others to be assigned by your instructor. For each solute, you will determine the molarity of each ion of a saturated solution at a number of different temperatures (at least five per solute) as follows:
- Obtain distilled water and measure out 20 mL into a 250 mL beaker.
- Get a foam cup (from Glassware > Other) ready.
- Heat the water to the desired temperature in the beaker, and then pour the water quickly into the foam cup.
- Add the desired solid into the cup until there is solid (as shown in the information tab on the left).
- “Filter” the solution by right clicking on the foam cup and selecting “remove solid”.
- Record the molarities of the cation and anion, along with the temperature of the contents of the foam cup (which will not be the same as the temperature of the water you had).
You can then use this information to create a van’t Hoff plot as described above, and then obtain ![]() and
and ![]() for this process.
for this process.
