3 Determination of the Concentration of a Solution of Sodium Hydroxide
Yu Kay Law
Purpose
To determine the concentration of an unknown hydrochloric acid solution using acid-base titration methods.
Expected Learning Outcomes
- Describe and perform an acid-base titration experiment using indicators to find the end point.
-
Solve stoichiometry problems in the solution phase.
Textbook Reference
Tro, Chemistry: Structures and Properties, 2nd Ed, Ch. 8.7.
Introduction
Acid-Base Chemistry
Definitions
There are a number of different definitions of acids and bases present. However, for most purposes, the Brønsted-Lowry definition of acids and bases are the most useful:
- An acid is a proton (H+) donor. Common examples include hydrochloric (muriatic) acid, citric acid, and ascorbic acid (Vitamin C).
- A base is a proton acceptor. Among the best known of these substances are hydroxides, such as sodium hydroxide; however, other bases exist. Examples of these include sodium bicarbonate (baking soda) and sodium hypochlorite (bleach).
Examples
When acetic acid (![]() ) is dissolved in water, the reaction is
) is dissolved in water, the reaction is
(1) ![]()
where you see that the acetic acid acid loses a proton and water, acting as a base, gains a proton to form the hydronium ion ![]() .
.
Acid-Base Neutralization Reactions
One of the most commonly observed and studied types of acid-base reaction is the acid-base neutralization reaction, where an acid is reacted with a base:
(2) ![]()
where the identity of the salt can be determined by taking the cation from the base and taking the anion from the acid.
Examples
If I react sodium hydroxide with hydrochloric acid, the cation is Na+ from the sodium hydroxide and the anion is Cl– from the hydrochloric acid:
(3) ![]()
The Titration Setup
A titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples in such a way that the exact volume of the two reactants are known. If the concentration of one of the two reactant solutions is known exactly, then one can determine the concentration of the second solution accurately.
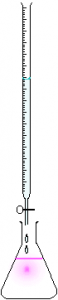
of the setup for
a titration.
A typical titration setup is illustrated in the figure below. A measured volume of the analyte – typically the acid – is placed in the Erlenmeyer flask at the bottom along with an indicator. The indicator is a compound that will change color when the reaction has completed to the end point. A volumetric pipet is typically used to measure out the exact amount of analyte needed.
A buret is used to deliver the titrant (typically the base in acid-base titrations). The titrant is added in dropwise such that the volume of titrant required to change the color of the indicator can be recorded to the nearest 0.01 mL. Therefore, we can determine the exact (or as close to exact as we can) volume of titrant required to react with the analyte.
In this experiment, you will use potassium hydrogen phthalate (![]() , KHP; molar mass = 204.2 g/mol) as an analyte, and sodium hydroxide (a base) will be used as the titrant. You will use phenolphthalein as the indicator. This is a monoprotic acid and the balanced chemical equation for this reaction is:
, KHP; molar mass = 204.2 g/mol) as an analyte, and sodium hydroxide (a base) will be used as the titrant. You will use phenolphthalein as the indicator. This is a monoprotic acid and the balanced chemical equation for this reaction is:
(4) ![]()
From the information obtained the concentration of the provided sodium hydroxide solution can be determined. Sodium hydroxide cannot be used as a primary standard readily, as it tends to be extremely hydroscopic and therefore the mass of sodium hydroxide used is not an accurate starting point for determining the concentration of base used. For this reason, we use KHP as a primary standard, with which the concentration of the sodium hydroxide solution can be determined accurately (and therefore act as a secondary standard).
The end-point – which is when the analyte is completely reacted with the titrant – is determined using phenolphthalein as an indicator. When the end-point of the titration is reached, the color of the solution changes from colorless to pink.
Procedures
You will use the Determine the concentration of an unknown HCl solution and Standardization of NaOH experiment (OLI Unit 3 – Module 11)
- You must prepare your notebook just like a regular face-to-face experiment and record all your data there in handwriting. At the end of the experiment, you are required to scan and submit your lab notebook entries.
- While there are directions on the OLI webpage, we have written new ones that are designed to allow you to repeat the experiment.
- Volumes from volumetric flasks and pipettes should have four significant figures.
- For each titration, you may want to try a rough titration first where you go through it quickly so you have a maximum volume it might be at, and then try it more carefully by starting off with adding NaOH to within a few mL before doing the titration in full. The rough attempt should be recorded in the notebook but can be ignored for the data analysis.
Preparation of a Solution of Potassium Hydrogen Phthalate
- Remove the KHP, a 250 mL beaker, and scale from the stockroom.
- Place a weigh boat on the scale and tare the balance. Be sure to record the entire reading on the scale.
- Transfer 7-9 g of KHP to the weigh boat. Note that the transfer mode is “Realistic”. Clicking once, quickly on the “Hold to Pour” button will transfer this amount. Record the mass of the KHP. Afterwards, transfer all of this into a 250 mL volumetric flask. (Note: the volume of a volumetric flask has four significant figures).
- Make up the solution to 250 mL in the volumetric flask with distilled water from the stockroom.
Standardization of the Sodium Hydroxide Solution
In this part, you will determine the exact concentration of the ~0.1 M sodium hydroxide solution using the potassium hydrogen phthalate solution you prepared earlier as a standard.
- Fill the buret approximately to the 5.00 mL mark with the NaOH solution.
- Using a volumetric pipet, measure out 25.00 mL of the potassium hydrogen phthlate solution and put this in a 250 mL Erlenmeyer flask.
- Put a few drops of phenolphthalein from the stockroom into the Erlenmeyer flask.
- Record the initial volume of sodium hydroxide in the buret.
- Overlap the buret on top of the Erlenmeyer flask so it is placed directly above the Erlenmeyer flask and is poised to deliver the solution.
- Add portions of the sodium hydroxide from the buret into the Erlenmeyer flask. As you near the desired concentration, be sure to add in the sodium hydroxide dropwise until the phenolphthalein indicator turns pink.
- Record the final volume in the buret.
- Empty the contents of the Erlenmeyer flask.
Repeat this part at least two more times. Do a fourth trial if the volume delivered from the first three trials are more than 0.2 mL apart.
Determination of the Concentration of the Unknown Hydrochloric Acid Solution
Repeat how you did the titration of KHP against sodium hydroxide, except using 25 mL of the unknown hydrochloric acid solution instead of 25 mL of the KHP solution.
Data Analysis
This laboratory experiment is rather complex to calculate and takes some time to do. Be sure to complete all calculations carefully. If you do not understand how to do these calculations, please be sure to consult your instructor or a mentor in the Math/Science Resource Center.
Determining the Concentration of Potassium Hydrogen Phthalate (KHP) Solution
Potassium hydrogen phthalate has a molar mass of 204.2 g/mol. Given the mass of the potassium hydrogen phthalate present and the volume of the volumetric flask (which you will need to convert into liters), you should be able to determine the molarity of the solution using the definition
(5) ![]()
Examples
If we dissolve 3.224 g potassium hydrogen phthalate (KHP) to form a solution of 100.0 mL, the molarity of KHP in this solution is
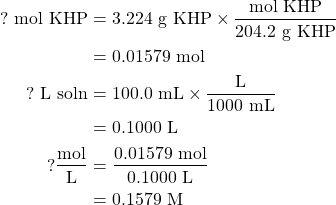
Determining the concentration of the 0.1 M sodium hydroxide solution
This can be solved as a solution stoichiometry problem. To do this, the first step is to determine the number of moles of sodium hydroxide present, and then divide by the volume of NaOH used.
Examples
10.00 mL of a 0.1523 M KHP solution requires 14.22 mL of sodium hydroxide to neutralize. Determine the concentration of the NaOH solution.
![Rendered by QuickLaTeX.com \begin{align*} ?\mbox{mol NaOH} &= 10.00 \mbox{ mL KHP} \times \frac{\mbox{L KHP}}{1000\mbox{ mL KHP}} \times \frac{0.1523 \mbox{ mol KHP}}{\mbox{L KHP}} \times \frac{1\mbox{ mol NaOH}}{1\mbox{mol KHP}} \\ &= 0.001523 \mbox{ mol NaOH} \\ ?\mbox{ L NaOH} &= 14.22\mbox{ mL} \times \frac{\mbox{L}}{1000\textrm{ mL}} \\ &=0.01422\textrm{ L}\\ \unit[?]{M} &= \frac{0.001523\textrm{ mol}}{0.01422\textrm{ L}} \\ &= 0.1071\textrm{ M} \end{align*}](https://iu.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-d789eb34926ab60373c68bb088af0bf4_l3.png)
Similar approaches can then be used to find the concentration of hydrochloric acid.

