9 Absorption Spectrum of Allura Red
Purpose
To determine the concentration of allura red (FD&C Red 40) in Kool-Aid.
Expected Learning Outcomes
After completing this experiment, students are expected to be able to
- Relate the color of a solution to its absorption spectrum. (LO 5)
- Measure the absorption spectrum of a substance. (LO 3)
- Make Beer’s Law plots and use the plot to find the concentration of a sample. (LO 3, 4, 6)
Textbook Reference
Tro, Chemistry: Structures and Properties, 2nd Ed, Ch. 8.2.
Introduction
Absorption of Light
As we saw in our discussion of spectroscopy related to our research project a few weeks ago, when light passes through a sample, some proportion of the light will be absorbed, depending on the electronic structure of the molecule[1] and the wavelength of the incoming light, while the rest of the light is transmitted. In the figure below, the incident (incoming) light intensity is [latex]I_0[/latex] and the transmitted light intensity (the light that is able to make it through the sample and out the other side) is [latex]I_t[/latex].
To quantify this, we note that – at a particular wavelength – given that the intensity of incident light is [latex]I_0[/latex], the intensity of light that goes through the sample is [latex]I_1[/latex]; the rest of this light is absorbed by the sample:
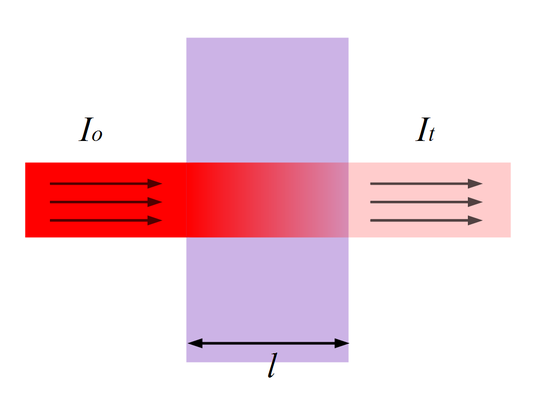
To quantify the amount of light that is absorbed, we define the absorbance ([latex]A[/latex]) as
\begin{equation}
\frac{I_1}{I_0} = 10^{-A} \to A = -\log_{10}\left(\frac{I_1}{I_0}\right)
\end{equation}
Note that the absorbance is unitless.
In this case, we can plot an absorption spectrum – a graph of the absorbance ([latex]A[/latex]) as a function of the wavelength of a sample:
The color of the solution will be the complementary color of the color of light being absorbed – which is the color opposite the wavelength of light in the color wheel:

Examples
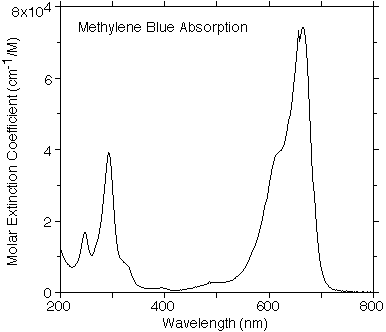
Methylene blue absorbs primarily at around 620 nm, which corresponds to orange on the visible spectrum. This is opposite to blue on the color wheel, and aligns with the solution.

The visible spectrum is as follows:
The absorbance of a solution can be measured using a spectrophotometer; details on how to use this is explained in Using Laboratory Equipment.
Beer-Lambert Law
It can be shown that for a solution with a concentration (molarity) of [latex]c[/latex],[2] the absorbance is related to this by
[latex]A = \varepsilon cl[/latex]
where [latex]l[/latex] is the path length (the thickness of the solution through which the light travels; this is typically reported in centimeters) and [latex]\varepsilon[/latex] is the molar absorptivity (with units of [latex]\mbox{M}^{-1}\mbox{cm}^{-1}[/latex]). The molar absorptivity varies with wavelength, and is a property of a particular substance at a given wavelength.
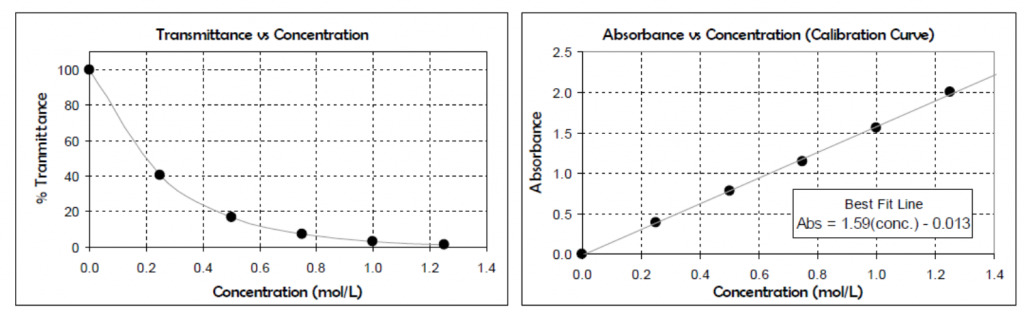
The molar absorptivity at a given wavelength can be found by producing a Beer’s Law plot (like on the right hand side of the figure above). To do this, solutions of different concentrations of the compound being studied are prepared and their absorbances at the chosen wavelength are plotted (along the y-axis) against the concentrations of these solutions (along the x-axis).
Based on this, the molar absorptivity ε can be found from the slope of the Beer’s Law plot. The slope of the Beer’s Law plot is equal to [latex]\varepsilon l[/latex]. Most cuvettes used in the laboratory have a 1 cm pathlength, and therefore in your experiment you can use this to find the molar absorptivity. Remember that typically Beer’s Law is set up with units such that the path length is in cm.
Using the molar absorptivity, we can determine the concentration of an unknown solution of the same compound[3] by measuring the absorbance of the sample at the same wavelength as was done for the standard solutions. Given the molar absorptivity found from the Beer’s Law plot, one can solve Beer’s Law to find the absorbance.[4]
This technique is very widely used in modern analytical chemistry and is one of the primary ways, for example, by which proteins and nucleic acids are quantified in the biochemical laboratory.
Dilution Calculations
Often, in chemistry, we start with more concentrated stock solutions and dilute these to get the concentration we want. This is a key skill that you should acquire. We do this quite often with concentrated drinks as well.
When we dilute a stock solution with a volume [latex]V_1[/latex] by a molarity [latex]M_1[/latex] with the solvent such that the final volume of the solution is [latex]V_2[/latex], the molarity of the diluted solution can be related to the other quantities by
\begin{equation}
M_1 V_1 = M_2 V_2 \label{306A:dilution}
\end{equation}
This equation could also be used to back-solve the concentration of a stock solution from that of a diluted solution. Be aware that this equation can only be used when you are diluting something (i.e. when you add water to a SOLUTION to decrease that solutions concentration). It cannot be used to calculate the concentration when you dissolve a SOLID (or a pure liquid instead of a solution) in water.
Example: if you take 32.6 mL of a 0.53 M NaCl solution and dilute it to 99.3 mL with distilled water (i.e. the total volume of NaCl solution + distilled water = 99.3 mL), we can calculate the molar concentration of the diluted solution using M1V2=M2V2. M1 = the molar concentration of the stock solution (0.53 M NaCl in this example). V1 = the volume of the stock solution that was used to make the diluted solution (32.6 mL in this example). M2 = the molar concentration of the diluted solution (what we’re trying to find in this example). V2 = the total volume of the diluted solution (99.3 mL in this example). When we plug these numbers in, we get 0.53 M NaCl * 32.6 mL = X M NaCl * 99.3 mL. This can then be rearranged to isolate M2: X M NaCl = (0.53 M NaCl * 32.6 mL)/99.3 mL = 0.17 M NaCl.
Procedures
- This experiment will be done in pairs.
- Be sure to refer to
- Using Standard Laboratory Equipment and your own notes from the week 3 experiment on calculating the density of water for information on how to use burets.
- Using Laboratory Equipment and your own notes from the 2nd week of the research project for information on how to use the GoDirect SpectroVis Plus spectrophotometers. You might also want to watch this video on how to use the SpectroVis to create a Beer’s Law plot.
Both of these are critical for this experiment.
- You will need to install Vernier Spectral Analysis (available for free from the App Store for your device, linked to from here) to use the spectrophotometer.
- For the SpectroVis Plus spectrophotometer, absorbances above 1 tend to make Beer’s Law break down. Consult your instructor if your solutions record an absorbance above 1.
- Use the same cuvette throughout the experiment.
- Keep the results of this experiment in your laboratory notebook as you will need this information in CHEM-C 126.
Chemicals Needed
Please pour the following amounts of chemicals into your own glassware.
- Stock solution of allura red: 35 mL. You will need to record the concentration of the stock solution from the bottle.
- Kool-Aid: 3 mL. You will need to record the concentration and flavor of the Kool-Aid from the bottle.
Obtaining the Absorption Spectrum for Allura Red
In this part of the experiment, you will use the standard Allura red stock solution to obtain the overall absorption spectrum of Allura red.
- Observe and describe the color and appearance of the solution.
- Following the directions in Using Laboratory Equipment, measure the absorption spectrum of the stock solution (using water to calibrate the spectrophotometer).
- Determine the best wavelength (i.e. the wavelength that gives the highest absorbance) at which you should measure the absorption values for the Beer’s Law plot, and record this wavelength and the absorbance at that wavelength in your notebook. Note that this wavelength will be slightly different for each group due to random error from the spectrophotometer itself.
- Save the absorption spectrum by selecting from the menu for the file name at the top left hand corner and enter “Export”. Save an image of the graph.
Preparation of Solutions for Beer’s Law plot
In this part of the experiment, you will prepare a series of diluted solutions to prepare a Beer’s Law plot.
- Rinse the buret with small portions of deionized water and then rinse with small portions of the stock solution. Fill the buret to the 25 mL mark (approximately) with the stock solution provided.
- Select “New Experiment” from the file name at the top left hand corner of your screen, and then select Absorbance vs Concentration (Beer’s Law).
- Enter the wavelength you selected in step 3 above.
- Measure the absorbance (click “measure”, then “keep”) with the SpectroVis, then enter the concentration of 5 standards with different concentrations of Allura red by diluting the stock solution an appropriate amount. You will need to use your results in the previous part of the lab on collecting the absorption spectrum of Allura red to come up with the approximate dilutions of the stock solution that you want to use so that the 5 solutions have an equally distributed range of absorbances from ~0.2 to ~1.0.
- Record the initial volume of solution in the buret, being sure to estimate the hundredths place.
- Carefully rinse your 10 mL graduated cylinder and empty it as best you can (just like you did in the Week 3 experiment on determining the density of water), but you may not be able to empty it completely; this will be okay as long as you have rinsed it carefully.
- Calculate the amount of stock solution you need to make ~10 mL of a diluted solution with approximately the desired absorbance. Deliver this volume of the stock solution into the 10 mL graduated cylinder using the buret.
- Record the final volume in the buret, being sure to estimate the hundredths place. The difference in the initial and final volumes is the volume delivered.
- Fill the graduated cylinder carefully with deionized water such that the final volume is just below the 10 mL mark on the graduated cylinder. Record the actual volume used to the correct number of significant figures.
- Use a piece of Parafilm to seal the top of the graduated cylinder. Invert the graduated cylinder about twenty times until the mixture is completely mixed.
- Use M1V1=M2V2 to calculate the exact concentration of this standard (M2) using the concentration of the stock solution (M1), your measured result from sub-step 4 (V1), and your measured result from sub-step 5 (V2).
- Measure the absorbance of this solution, entering the correct concentration from sub-step 7 (do NOT enter the concentration in scientific notation, use the full decimal form – e.g. 0.0035 instead of 3.5 x 103). Check that the graph matches what you would expect from Beer’s Law (i.e. make sure all the data points are approximately in line with each other). If your graph looks curved, or the data points don’t line up, consult with your instructor as you will need to double check your concentration calculations, remake your dilutions and/or make new dilutions at different concentrations to make sure the absorbance isn’t >1.0.
Obtaining the Molar Absorptivity
- On Vernier Spectral Analysis, click on the Graph tool icon on the bottom left of the graph and select Apply curve fit. Select the linear function and apply that fit.
- It will show the parameters for the fit. Record the information in the box that pops up (you might need to scroll down to see all the information in the box).
- In preparation for exporting an image of the plot, move the box containing the curve fit data somewhere that doesn’t block the data points or the best-fit-line on your graph.
- Export the plot as you did in step 4.
- If you can’t get the function to work in the Vernier Spectral Analysis software, you can also plot the absorbance vs the concentration using Excel or Google Sheets and create the graph and the best-fit-line that way. See your instructor if you need help.
Determining the Concentration of Allura Red in Kool-Aid
Write down the identity of the Kool-Aid (flavor/type), the concentration of Kool-Aid powder and determine the concentration of Allura red in this drink by measuring its absorbance at the same wavelength used to create the Beer’s Law plot in the previous part of the experiment. If the absorbance is greater than 1, dilute the solution appropriately so that the resulting solution has an absorbance of less than 1 and greater than 0.1 and record (a) how you diluted it (including the volume of Kool-Aid you used and the volume you diluted it to, reporting the proper number of sig figs, and the glassware you used to measure out those volumes) and (b) what the absorbance (as measured by the SpectroVis; include the wavelength measured at) and concentration (in g/mL) of Kool-aid powder (as calculated using M1V1=M2V2, keeping in mind the M1 for the Kool-Aid has nothing to do with M1 for the Allura red stock solution) was at the end (i.e. in the cuvette). It is expected that you will need to make multiple dilutions of the Kool-Aid before you find one that gives an absorbance of <1 and >0.1.
Waste Disposal
All solutions from this experiment can be disposed of down the drain.
- The discussion on this is rather complex and is well beyond the scope of this course, but it is related to 2 topics taught in CHEM-C 105: atomic absorption spectroscopy and resonance and some discussion of how this impacts what wavelengths a molecule absorbs is had in the CHEM-C 344 organic chemistry laboratory course. ↵
- While we will use molarity for this purpose in this class, in principle you can use any concentration unit. It will just alter the units/numerical value of ε. ↵
- In the same solvent, in principle, though the absorption spectrum doesn't vary too much as a function of solvent in many cases. ↵
- You should, however, be aware that Beer's Law only works for relatively low concentrations. Beyond an absorbance of about [latex]A = 1.5[/latex], Beer's Law breaks down. ↵

