12 Alcohol: Production by Fermentation and Distillation
Purpose
To prepare ethanol (alcohol) through fermentation and purify this using simple distillation.
Expected Learning Outcomes
- Describe the process and principles of fermentation.
- Purify a substance using distillation.
- Evaluate the purity using experimental data.
Introduction
Alcohols
In organic chemistry, molecules are classified partly based on the functional groups. A functional group is a particular bonding arrangement of atoms which behave as a unit chemically. Common examples are given in McMurry, OpenStax Organic Chemistry, 10th Ed, Ch. 3.1. Of these, one of the most common is the alcohol group, where -OH is bonded to a carbon backbone. The most commonly found alcohols are ethanol (C2H5OH, found in food and drink), methanol (CH3OH, found in copier toner), and 2-propanol (CH3)2CHOH, commonly known as isopropyl alcohol).
In this experiment, you will prepare ethanol using fermentation and purify this ethanol using distillation.
Preparation of Ethanol
Fermentation
Alcoholic fermentation has been familiar to the human species since prehistoric times, yet it was not until after 1857 that its cause was discovered. In that year, Louis Pasteur showed that the cause was microscopic organisms. The overall reaction of fermentation of sucrose is:
[latex]\textrm{C}_{12} \textrm{H}_{22} \textrm{O}_{11} + \mbox{H}_2\mbox{O} \to 4\mbox{C}_2\mbox{H}_5\mbox{OH} + 4\mbox{CO}_2[/latex]
Fermentation is of considerable commercial importance. For example, the production of alcoholic beverages and the baking industry both utilize fermentation. More recently, ethanol mixed with gasoline has been used as a fuel to extend the use of fossil fuels. One problem with the use of fermentation is that the maximum ethanol concentration that can be reached is approximately 12% (by volume). The reason is that the yeast cells will die at higher concentrations of ethanol.
Distillation
Distillation is the process in which a liquid is boiled and its vapor is recondensed elsewhere to separate it from higher boiling substances. When you vaporize a liquid, each component would have a different partial vapor pressure given by Raoult’s Law
[latex]P_A = \chi_A P_A^\circ[/latex]
where [latex]P_A^\circ[/latex] is the vapor pressure of the pure liquid, and [latex]\chi_A[/latex] is the mole fraction.[1] The more volatile compound would have a higher vapor pressure for the pure liquid, and would therefore form a larger proportion of the vapor phase.
Examples
The liquid condensate is then collected. Since this was condensed from the vapor phase, it would have the same composition as the vapor phase (and hence contain more of the more volatile component.
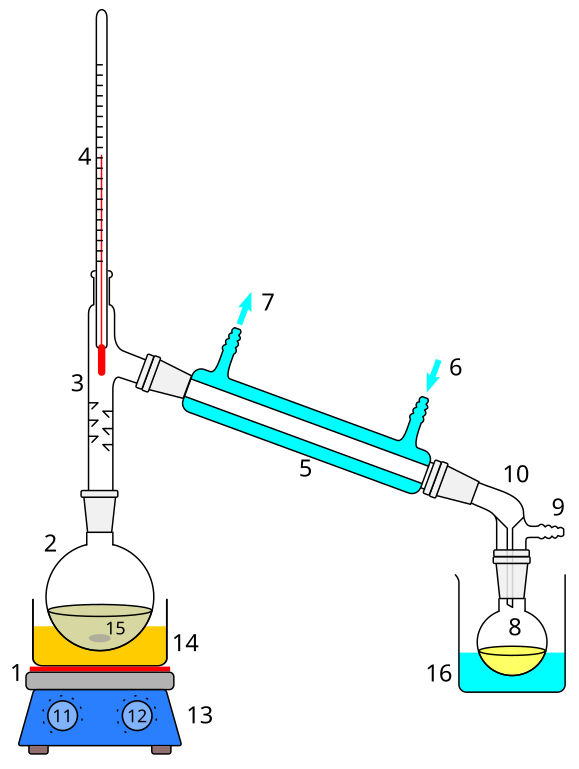
The technique requires a heat source, a vessel containing the mixture to be separated, a short vapor pathway to a cooled condenser and a receiver for collecting the condensed liquid. A typical laboratory distillation apparatus is shown on the following figure. The condensed liquid collected is referred to as the distillate.
Determining the Alcohol Content of the Mixture
To determine the alcohol content of the mixture, you need to be able to measure the density of the liquid carefully. From this, we can find the alcohol content of the product as well as we can. This is in fact the basis of some of the methods for determining the alcohol content of alcoholic drinks. These values can be found in Table 2-113 (p. 2-118) of Perry’s Chemical Engineers’ Handbook.
This will give you the alcohol content in percent by mass (same as the percent by weight). To convert this to percent by volume, you can use approaches found in Tro, Chemistry: Structures and Properties, 2nd Ed, Ch. 13.5. This will be done for you on the report form.
Experimental Procedure
As it takes time for the yeast to ferment, you will set this up the first week of class and place in your drawer in lab. In the second week, you will complete the bulk of this experiment.
Week 1: Preparation of the Fermentation Mixture
The prelab quiz will be done the following week. Please consult your instructor if both you and your lab partner are absent for the fermentation process
Special Equipment/Supplies
- Chemical label
- Sharpie
Procedure
- Appropriately label a 250 mL Erlenmeyer flask with the contents (see description below), your names, and today’s date.
- Weigh out approximately 30 g of sucrose.
- Transfer the sucrose to the 250-mL Erlenmeyer flask and add 150 mL of deionized water.
- Swirl the flask to dissolve the sucrose and add 30 mL of a solution of Pasteur’s salts (a mixture of potassium phosphate, calcium phosphate, magnesium sulfate and ammonium tartrate in water).
- Obtain 0.5 g of yeast and add the yeast to the flask
- Mix everything thoroughly. Stopper the flask with a cotton ball.
- Label the flask and place it in the designated tray provided by your instructor.
Waste
All solutions containing mineral oil, calcium hydroxide, or Pasteur’s salt shall be disposed of as waste.
Week 2: Distillation and Characterization of Ethanol
Special Equipment/Supplies
- 100 mL round-bottomed flask
- Alcohol thermometer
- Condenser
- Volumetric pipet
Procedure
- Observe the flask containing the fermentation mixture and the test tube containing the lime water, and record your observations.
- Determine the mass of a small, empty beaker.
Filtration of the Fermentation Mixture
You will use a vacuum filtration setup to filter the fermentation mixture.
- Following the directions in the back matter, set up and vacuum filter approximately 80 mL of the fermentation mixture. Try not to stir up or incorporate into the mixture any of the residue from the bottom of the fermentation mixture.
- Mix the filtered sample well and pipette into the small, empty beaker using a volumetric pipet 5 mL of the filtered fermentation mixture and determine the mass of this aliquot. Empty and allow the beaker to dry.
Distillation
- Measure out approximately 50 mL of the filtered fermentation mixture into a graduated cylinder. Pour this into the round-bottomed flask. Additionally, add two boiling chips to the distilling flask.
- Position the thermometer so that the top of the alcohol bulb is just below the side arm and assemble the distillation apparatus as shown on the figure above. Connect the rubber tubing connected to the lower end of the condenser to the water supply and place the other end of the rubber tubing connected to the upper end of the condenser in the sink. For heating, you will use a heating mantle (available from the back of the lab room).
- Turn on the water supply. to ensure that there is a continuous flow of water through the condenser, and that the outer jacket is almost completely filled with water. Check with your instructor to ensure that the distillation setup is assembled correctly before proceeding.
- Heat the sample using a heating mantle. When the vapor temperature begins to approach the boiling point and distillate begins to appear, collect the distillate.
- At the boiling point, the mixture’s temperature will remain constant. Record this temperature. Stop collecting liquid when the temperature begins to rise significantly above the boiling point.
Characterizing the Product
- Describe qualitatively each fraction obtained (color and smell – being careful to waft the fumes to your nose rather than to smell the product directly) as well as the residue in the round-bottomed flask.
- Weigh a small, empty beaker. For each fraction as well as the residue, pipette into the small, empty beaker using a volumetric pipet 5 mL of the filtered fermentation mixture and determine the mass of each aliquot. You must measure the mass of the beaker before and after putting the alcohol into the beaker.
Waste
The waste from this experiment can be washed down the drain with plenty of water.
- The number of moles of A divided by the total number of moles of particles. ↵

