11 Conductivity of Aqueous Solutions
Purpose
To observe the difference in conductivity between strong, weak, and non-electrolytes.
Expected Learning Outcomes
After completing this experiment, students should be able to
- Classify solutes as strong electrolytes, weak electrolytes, and non-electrolytes in terms of the degree of dissociation and conductivity. (LO 3, 4)
- Describe and explain the relationship between the concentration of electrolytes and the conductivity of solutions. (LO 5)
Textbook Reference
Tro, Structures and Properties, 2nd Ed, Ch. 8.4.
Introduction
The Behavior of Compounds When Dissolved in Water
In water, for reasons that will become more apparent in Chapters 11 and 13, ionic compounds and molecular compounds behave differently.
Ionic Compounds
When ionic compounds dissolve in water,[1] they dissociate into their constituent ions when they dissolve in water.
Examples
When sodium chloride is dissolved in water, the crystal structure breaks apart such that sodium ions and chloride ions are dissolved separately in water
\begin{equation}
\ce{NaCl}(s) \overset{\ce{H2O}}{\rightleftharpoons} \ce{Na+} (aq) + \ce{Cl-}(aq)
\end{equation}
Note that polyatomic ions will remain as discrete units; when dissociation occurs, covalent bonds will not be broken. You will also need to pay attention to the number of ions present.
Examples
When sodium sulfate is dissolved in water, the sulfate ions do not break down separately. Instead, we will get sodium ions (of which there are two) and sulfate ions.
\begin{equation}
\ce{Na2SO4} (s) \overset{\ce{H2O}}{\rightleftharpoons} 2\ce{Na+} (aq) + \ce{SO}_4^{2-} (aq)
\end{equation}
It is important to note that
- Since there are two sodium ions in [latex]\ce{Na2SO4}[/latex], there will be two of them in solution.
- The sulfate ion, [latex]\ce{SO}_4^{2-}[/latex], does not dissociate further as it is held together by covalent bonds. Therefore, you expect to see sulfate ions present together.
Molecular Compounds
These are held together by covalent bonds and therefore will not dissociate when dissolved in water.
Examples
While this is generally the case, an exception is found for acids and bases. As explained in your textbook, acids and bases undergo ionization and therefore will form charged particles in solution.
Of these, some of them will ionize to completion (i.e. the yield of ions from the original acid/base molecules is close to 100%). These are referred to as strong acids or bases.
Examples
Hydrochloric acid (HCl) is a strong acid and therefore will ionize completely when dissolved in water}:[2]
\begin{equation}
\ce{HCl} (aq) \to \ce{H+} (aq) + \ce{Cl-}(aq)
\end{equation}
On the other hand, most acids and bases do not ionize completely; only a small proportion of ions are ionized, as illustrated for acetic acid below. While some of the molecules are split up into H+ and [latex]\ce{C2H3O2-}[/latex] ions, most of the acetic acid molecules remain intact and the O-H covalent bond is not broken.
We will explore this in more depth in CHEM-C 106.
Electrolytes and Non-Electrolytes
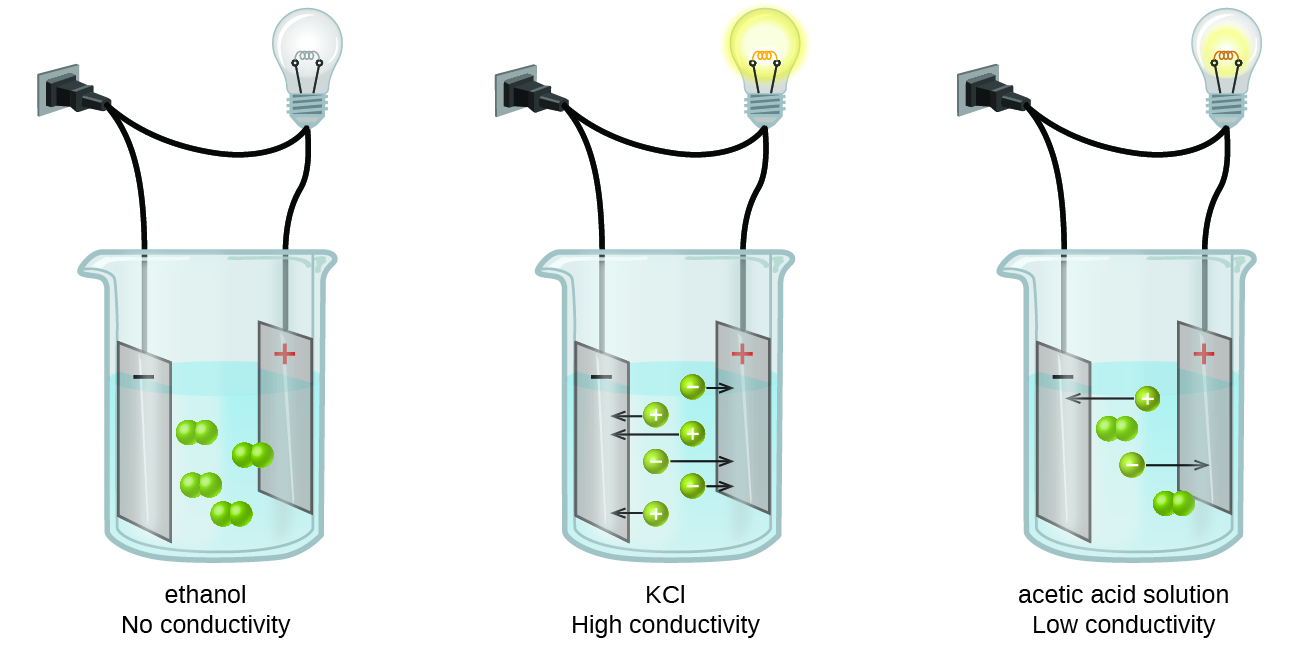
It is known that while some liquids will conduct electricity to various extents, other liquids are shown to not conduct electricity. We can also distinguish between these within the realm of aqueous solutions – solutions where the solvent is water.
- Non-electrolytes do not conduct electricity when dissolved in water. These compounds are molecular and are not acids or bases and hence will not dissociate in water.
- Electrolytes contain ions, and since there are freestanding charged particles in the solution, they conduct electricity to varying extents. Since the mobility of different types of ions in solution vary significantly, so will the conductivity. Regardless, two different factors that we understand will affect the conductivity significantly.
- The greater the concentration of ions present in a solution, the greater its conductivity.
- We can classify electrolytes as strong or weak electrolytes:
- Weak electrolytes do not dissociate/ionize much, they tend to have significantly lower ion concentrations and therefore they do not conduct as much as strong electrolytes.
- Strong electrolytes dissociate/ionize completely; none of it would not dissociate and they tend to conduct well.
Conductivity Meters: What The Reading Means
In this lab, we will use pen-style conductivity meters designed to measure the amount of trace ionic impurities in water samples. These are calibrated for this purpose such that the conductivity is converted into parts per million of dissolved solids
\begin{equation}
\mbox{parts per million} = \frac{\mbox{g dissolved solids}}{10^6\mbox{ g solution}} = \frac{\mbox{mg dissolved solids}}{\mbox{kg solution}}
\end{equation}
Different solutes will lead to different amounts of conductivity given the same concentration of solute. For the purpose of calibration, the meters are calibrated based on the expected conductivity for sodium chloride. The value reported is therefore only a relative measure of the concentration of dissolved ionic compounds in solution.
This is not critical for this experiment. We will simply make qualitative measurements in this experiment, treating the values reported as relative measurements of conductivity.
Procedures
- This experiment will be done in pairs, but each student must submit his/her own report.
- You need approximately 10-15 mL of each solution except for 0.0050 M hydrochloric acid, which you will need 75 mL of.
- This is the key assignment for general education assessment; this will need to be completed during the lab period and scanned/uploaded to Canvas for submission.
- Each pair will obtain a conductivity meter and rinse the conductivity meter carefully with deionized water, and then place the conductivity meter in a beaker of deionized water.
The value on the display is the nominal concentration of total dissolved solids (TDS), as discussed above. For this experiment, this is the value for the relative conductivity that we will record.
Be sure in each measurement that the fluid level of the probe is above the “MIN” marking and below the “MAX” marking. The easiest way of doing this is to fill test tubes so that they are about two-thirds filled before placing the probe into the sample.
Relative Conductivity of Different Reagents
- Record the relative conductivity for deionized/distilled water.
- Dispense into a test tube until the level of the liquid is a little over half way up the test tube and record the relative conductivity for the following reagents:
- ethanol (C2H5OH)
- 0.0050 M solutions of each of the following reagents
- sucrose (C12H22O11)
- hydrochloric acid (HCl)
- nitric acid (HNO3)
- potassium hydroxide (KOH)
- potassium chloride (KCl)
- potassium nitrate (KNO3)
- calcium nitrate (Ca(NO3)2)
- iron(III) nitrate ({Fe(NO3)3)
- ammonia (NH3)
- acetic acid (HC2H3O2})
- 1 M acetic acid
Relative Conductivities of Different Concentrations of Hydrochloric Acid
- Prepare about 50 mL of solutions containing the following concentrations of hydrochloric acid by dilution from the 0.0050 M HCl solution. You may use a graduated cylinder to measure out the volumes of the solutions. Measure the conductivity of each of these solutions.
- 0.0010 M
- 0.0020 M
- 0.0030 M
\begin{equation}
M_1 V_1 = M_2 V_2
\end{equation}
Waste Disposal
All waste in this experiment except for iron(III) nitrate can be disposed of down the drain with copious amounts of water. Iron(III) nitrate will be collected in a waste beaker for disposal.
- Not all do, as explained in Tro, \textit{Chemistry: Structures and Properties}, 5th Ed, Chapter 8.4. ↵
- Yes, I know I should probably have mentioned it as H3O+ or even fancier things, since H+ (or even H3O+) doesn't really exist. Dr. Law's nose is reaching across Whitewater Hall and growing every year. However, let's leave it like this for now. Simplicity helps a lot of the time. ↵

