6 The Formula of a Metal Oxide
Purpose
To determine the formula for magnesium oxide through a combustion experiment.
Expected Learning Outcomes
Through completing this experiment, student should be able to
- competently use a Bunsen burner and crucible/lid for gravimetric analysis. (LO 3)
- determine the empirical formula of a compound from the experimentally determined composition of the compound. (LO 4)
Textbook Reference
Tro, Chemistry: Structures and Properties, 2nd Ed, Ch. 4.11
Introduction
As you may know from reading the textbook, binary ionic compounds take on the same formula as their empirical formula.[1] Therefore, in principle, if we know the composition by mass of each compound, you can find its empirical formula – and hence find its formula.
In this experiment, you will be provided with some magnesium metal and will burn it with excess oxygen from the air. The reaction is:
[latex]\mbox{magnesium} + \mbox{oxygen} \to \mbox{magnesium oxide}[/latex]
The Law of Conservation of Mass states that the mass of magnesium plus the mass of oxygen used in the reaction must equal the total mass of magnesium oxide. Therefore, by determining the mass of the initial amount of magnesium used and the final mass of the magnesium oxide, you will be able to determine the mass of oxygen in magnesium oxide.
Based on the mass of magnesium and the mass of oxygen in the compound, you should be able to determine the formula for magnesium oxide.
Procedures
- This experiment is to be completed individually.
- This is a relatively long experiment with quite a bit of downtime; students should therefore prepare to work on something else while waiting for the reaction/procedure to complete.
- The biggest problems students will run into with this experiment is breaking the crucible and/or its lid. You will need to restart the experiment from the beginning if you break the crucible and/or crucible lid since that will have a major impact on your mas measurements.
- Never leave the Bunsen flame unattended! If you must go somewhere, please turn the Bunsen flame off or move it away from the crucible.
- Do not look directly into the crucible while it is hot, if possible. There is very bright light inside the crucible (from the combustion of magnesium).
- Do not weigh a hot crucible; wait for the crucible to cool down before weighing.
- Review carefully the directions under Using Standard Laboratory Equipment for using a Bunsen burner. Successful completion of this experiment requires you to have a good grasp of how to use a Bunsen burner and be able to control the flame carefully.
Special Equipment Needed
This is found on the carts at the side of the lab room near the fume hoods.
- Crucible + crucible lid
- Crucible tongs
- clay triangle
- wire gauze
Preamble: Manipulating the Crucible

For the crucible, open the crucible tongs and hold the crucible right between the bowed parts in the middle of the tongs, with the tips of the tongs pointing upwards. Then, you can hold the crucible carefully and move it slowly from where it is to where you want it to be.
For the crucible lid, place the top of the lid such that the tong tips hold the “handle” part of the crucible lid.
Once you have the crucible or crucible lid held in your tongs, you are ready to move the component. Hold a wire gauze horizontally (flat) with your other hand, and then lift the crucible or crucible lid above the wire gauze and move that part where you want it. The wire gauze is intended to potentially catch a dropped crucible if you lose control.
Preliminary Steps
Ensure that your crucible is as clean as possible. Burnt residue cannot usually be removed; however, as long as it doesn’t undergo further combustion it will not affect the experiment. Therefore, heat the crucible to ensure that all of the impurities have undergone combustion.
- Set up a ring stand, with a clay triangle on it.
- Set up a Bunsen burner underneath the clay triangle.
- Turn on the flame and set it up so that the hottest part of the flame (remember, this would be the tip of the inner blue cone) sits where the crucible would sit. If it isn’t right, adjust the position of the ring until this is the case.
- Place the crucible and lid in the ajar position as illustrated below and heat the crucible for five minutes under an intense flame (the crucible should glow red during this time).
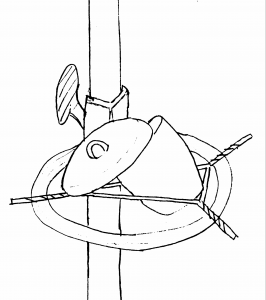
- Remove the crucible from the flame and allow for it to cool, remembering to follow the procedures described above.
- After the crucible and lid have cooled to room temperature, determine the mass of the crucible and lid. It is okay to determine the mass without the lid, but you will need to make sure that you consistently not include the lid when weighing in this case.
Heating the Magnesium
In this part, you will burn the magnesium and convert it into magnesium oxide as described above
- Take a strip of magnesium about 20 cm long. Sand it down thoroughly with sandpaper to remove all traces of corrosion (i.e. until it is shiny everywhere on both sides). Use the tip of your fingernail to apply extra pressure through the sand paper to remove any stubborn spots of corrosion.
- Loosely curl the strip of magnesium around a stir rod to obtain a helical shape (like a curly fry) that will maximize surface area and exposure to air while still fitting in the crucible. We want as much of the magnesium exposed to air as possible while still having all of the magnesium in the bottom half of the crucible.
- Place the reel of magnesium into the crucible (if need be, cut the reel into 2-3 pieces with a pair of scissors so that it’s all at the bottom of the crucible). Reassemble the crucible on the balance and determine the mass of the crucible with the magnesium reel in it. Check the mass of the magnesium: it should be about 0.15 g, but it doesn’t have to be exactly 0.15 g so long as you know exactly what the mass is. If the mass is significantly too low or too high (±0.03 g), add/remove magnesium until the right amount is present.
- Place the crucible on the clay triangle with a closed crucible lid, and heat the crucible relatively gently (with a medium amount of heat) for five minutes. During this time, you should open the crucible lid about every 30 seconds briefly to allow oxygen in.
- Cover the crucible and heat it strongly for about 35 minutes, with the bottom of the crucible being placed directly in the hottest part of the Bunsen flame (the tip of the inner blue cone, as illustrated in Using Standard Laboratory Equipment). To control the level of heat, change amount of gas (much safer than adjusting the level of the crucible). The exact amount of time you heat the crucible isn’t important, what matters is that you have fully oxidized all the magnesium metal. In most cases, the magnesium metal isn’t fully oxidized until around 35 minutes (but occasionally it will be fully oxidized after 20 minutes, so you might want to check every 5 minutes starting at the 20 minute mark).
- Lift the cover and observe the burning embers. You should see if any metal remains; it should have turned into white ash. If it hasn’t yet, then then re-cover the crucible and allow the contents to heat for another 15 minutes.
- When the contents appear to have completely converted to its oxide (white ash), remove the crucible lid and the crucible from the ring stand and place it on the lab bench. Call over the instructor to check to see if the reaction has gone to completion. If not, continue the heating process as advised by your instructor.
Converting Magnesium Nitride to Magnesium Oxide
Unfortunately one source of error is that at high temperatures magnesium can react with nitrogen (in the air) to form magnesium nitride. This can be resolved by adding water to the mixture, which would convert the magnesium nitride to magnesium oxide:
[latex]\mbox{magnesium nitride} + \text{water} \to \text{magnesium oxide} + \text{ammonia}[/latex]
- Add about 10 drops of deionized water into the crucible.
- Place the crucible back onto the clay triangle, and heat the crucible first for 2 minutes under medium heat, followed by 10 minutes under strong heat. Make sure that the crucible is held slightly ajar as illustrated above. This process will ensure the reaction is complete and that all added water is dried out.
- Remove the crucible from the flame and turn off the Bunsen burner.
- Wait for the crucible to completely cool and determine the mass of the crucible and its contents as before.
- Tip all of the contents of the crucible onto a paper towel (and throw the paper towel away). Wash,[2] dry, and replace the crucible back to the container where you found it.
Data Analysis
Remember, the formula for this compound must take the form [latex]\text{Mg}_x\text{O}_y[/latex], where x and y should be relatively small integers.
To do this, you need to first determine the mass of magnesium and oxygen in the crucible, recognizing that due to the Law of Conservation of Mass,
[latex]\mbox{mass of oxygen} = \mbox{mass of magnesium oxide} - \mbox{mass of magnesium}[/latex]
and therefore, using the molar masses of magnesium and oxygen, you should be able to determine the number of moles of magnesium and oxygen.
Then, calculate the ratio
[latex]\frac{\mbox{moles of magnesium}}{\mbox{moles of oxygen}}[/latex]
Report this number to three decimal places (regardless of significant figure rules).
Based on this ratio, you should be able to find the decimal close to a simple fraction, and hence find the formula for magnesium oxide.
Examples
Suppose we find that
[latex]\frac{\mbox{moles of magnesium}}{\mbox{moles of oxygen}} = 1.24[/latex]
In this case, the ratio is very close to [latex]\frac{5}{4} \equiv 1.25[/latex], and hence there are five moles of magnesium for every four moles of oxygen. As a result, the formula would be Mg5O4.
- This is not true of ionic compounds containing polyatomic ions, although the same principle holds. For polyatomic ions, however, we're looking for the lowest common denominator for the combination of ions. This might not lead to the same result as the empirical formula. For example, mercury (I) chloride has a formula of Hg2Cl2, but its empirical formula would be HgCl. ↵
- It is sometimes impossible to get rid of all residue - that's fine, don't worry about it as long as all loose particles are removed. ↵

