5 Research Project: Pigments in Plant Leaves
Purpose
To determine the composition of pigments in different types of plant leaves.
Expected Learning Outcomes
- Extract, separate, and characterize compounds from a mixture.
- Describe the purpose of analytical separations.
- Conduct thin-layer chromatography (TLC), column chromatography and high performance liquid chromatography (HPLC).
Reading Assignment
The focus of this experiment is on liquid phase extractions/separations. While separations are mentioned briefly in Tro, Chemistry – Structures and Properties, 2nd Ed, Ch. 1.2, the following readings from Nichols, L., Organic Chemistry Lab Techniques will help you understand this experiment:
In addition, we will introduce HPLC (high performance liquid chromatography. Please read Barkovitch, M., High Performance Liquid Chromatography and watch the following video produced by the Royal Society of Chemistry:
About This Project
So far, most of the experiments we have done have definite, well known answers, and we’ve been doing experiments that either (a) confirm what you’ve learnt or (b) illustrate concepts from the lecture course. We will continue to do those experiments, not least because the combination will support your learning and help you develop laboratory skills.
However, in real life, we often need to study problems with no well-defined solution or problem. We are dealing with unknown problems.
As you may know, there are a number of pigments in plant leaves (that make it the color they are) which are critical to photosynthesis.[1] However, there is a mixture of them, and as leaves have different colors, how would the pigments vary – what pigments are there, and how much? We will do this using two forms of column chromatography as explained below.
Chemical Principles
Mixtures
Most of the substances we find in nature (wood, air, petroleum, etc.) are not pure but are instead more or less complex mixtures. Some of these mixtures require little or no modification before being put to practical use, but many of them must be at least partially separated into their components before they can be used to the best advantage.
Examples
- Crude oil is a mix of different hydrocarbons (compounds formed from carbon and hydrogen) that is separated into different components as shown below.
- Hard water is “softened” by separating out dissolved calcium carbonate (limescale) from water.
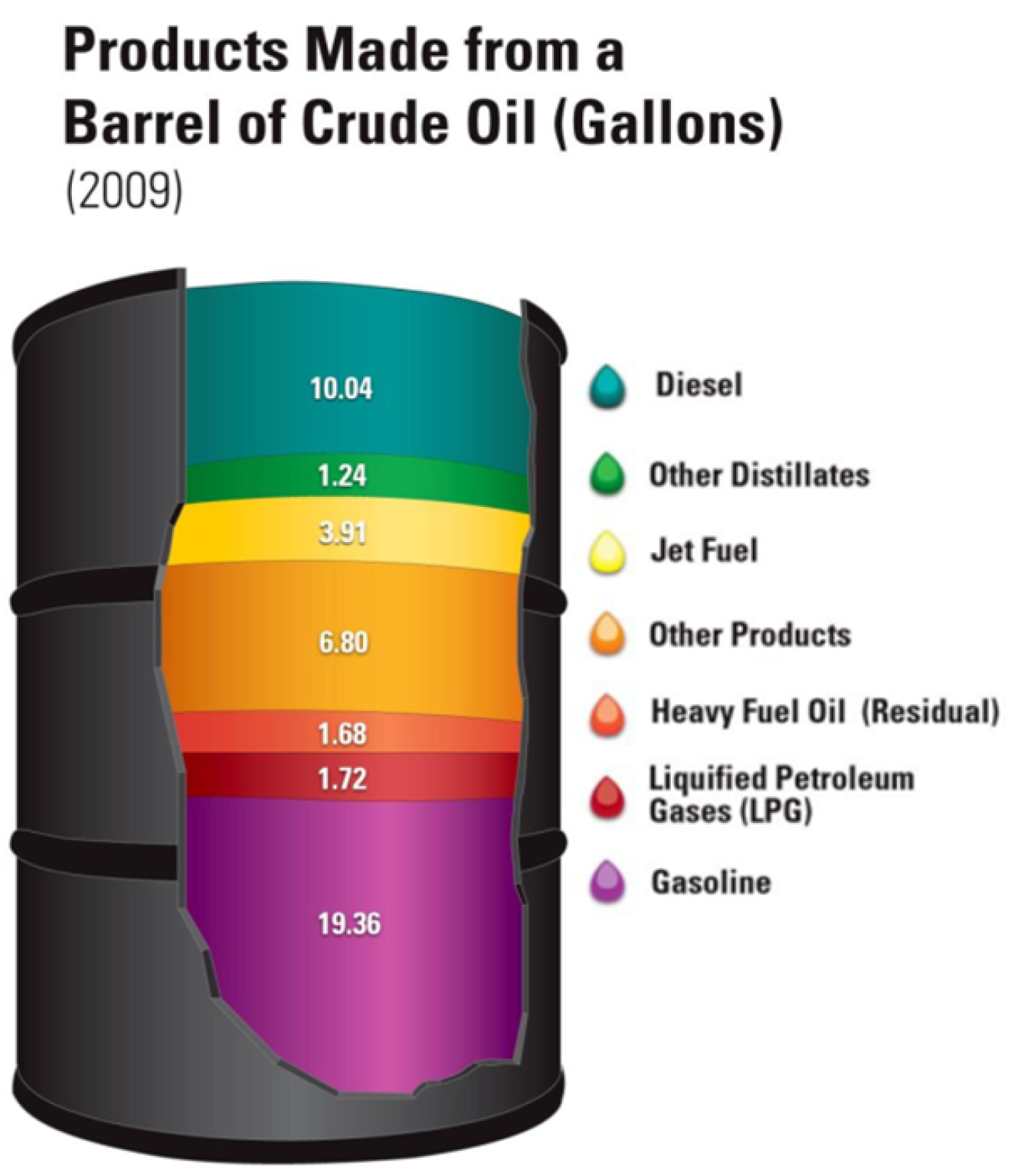
There are a number of chemical and physical processes used to separate mixtures but two of the most important for scientists today are the closely related techniques of extraction and chromatography.
Separation Method 1: Extraction
Extraction is the process of separating different substances by their differing solubilities in the same solvent (or solvent mixture).
Examples
When coffee is made using either a percolator or a drip-type coffee-maker, the hot water acts as a solvent to dissolve caffeine, tannic acid, and a large number of other chemical compounds, leaving the coffee grounds undissolved. This process is an extraction because of the differing solubilities of different substances: the caffeine and tannic acid are much more soluble in hot water than other components of the coffee grounds are (cellulose, for example).
Coffee-making illustrates the main requirements for a successful extraction:
- The mixture to be separated must be thoroughly mixed with the solvent (for example, by the “percolating” action).
- Other components must not dissolve in the solvent.

For a successful extraction we must find a solvent (or solvent mixture) that does a good job of dissolving the components we want but leaves undissolved components we don’t want. Since the solubility of most solids and liquids goes up with increasing temperature we can sometimes get the best results by using a hot solvent for the extraction. This is true for instance for most of the substances we want in our coffee, so we always start with very hot water (even if we are planning to end up with iced coffee). Other ways include using different solvents.
Separation Method 2: Chromatography
Chromatography is a general technique that has several points in common with extraction; for example, differing solubilities of different substances play a part in both techniques. Chromatography differs from extraction in that this always involves the movement of one phase (the “mobile phase”) past a non-moving phase (the “stationary phase”) to bring about the separation. Conditions are set up so that different components of the mixture are carried along with the mobile phase at different speeds as they pass through the stationary phase.
Examples
Different inks can be separated into different compounds/pigments using various forms of chromatography.

The big idea here is that there are two substances in which a substance can be dissolved:
- The stationary phase is a substance that is fixed along the entire structure where chromatography is conducted.
- The mobile phase which is typically a liquid or gas, and can move through the stationary phase.
Depending on the nature of the two phases and the substance concerned, there are varying ratios of solubility between the mobile phase and the stationary phase for different compounds. When a substance is more soluble in the mobile phase, it would tend to move along with the solvent front (the point where pure solvent has flowed to). Conversely, when a substance is more soluble in the stationary phase, then it would tend to stay on the stationary phase and not move.
Examples
For the inks on the TLC plate above, the extent to which the sample is soluble in the ethanol:water mixture relative to the silica (stationary phase, surface on the TLC plate) determines how far it migrates. Relatively speaking, the yellow ink is more soluble in ethanol:water (relative to silica) compared to the black ink.
While TLC is useful for quickly analyzing a sample and determining what compounds are present in that sample, it is very difficult to remove compounds from a TLC plate, thus it is not generally used to purify compounds. If you want to be able to collect the components of a mixture after separating them, column chromatography is more useful. In this technique, you place the stationary phase (for this experiment, silica gel) in a column (which is constructed using a glass, disposable Pasteur pipet). The sample is then loaded, and mobile phase is loaded to drive the sample until it elutes out of the bottom of the column. Each component is therefore isolated from each other and can be studied using absorption spectroscopy. This is the main method used to purify, among other things, proteins for biochemical experiments.

High Performance Liquid Chromatography (HPLC)
In this course, we have previously described how chromatography can be used to separate different components in a mixture. However, better separations can be made with columns created using specialized micrometer-sized particles (often silica). As the size of the particles decreases, the separation of the column improves.
However, with such fine particles the column would not function under room pressure. As such, high performance liquid chromatography (HPLC) instruments operate by forcing the solvent through the column under high pressure. In each case, the difference in affinity of solutes between the mobile and stationary phases is exploited to separate the substances.
While different column resins exploit different types of separation, one of the most common approaches to separation is by polarity of the solute. One of the most prevalent resins is that for reverse-phase HPLC (RP-HPLC), where a non-polar stationary phase is used in conjunction with a polar mobile phase. The most common resins for reverse phase HPLC are the octyl (C8) and the octadecyl (C18) columns.
The sample will then be detected by some means (often, UV-vis absorption) and a chromatogram is formed. Such a chromatogram is a plot of the signal as a function of time elapsed since the sample is injected.

Absorption Spectrum
Depending on the chemical structure of the molecule, different molecules can absorb light of different wavelengths. The color of the compound is the complementary color of the color of light absorbed – as seen in the color wheel, where the color observed is on the opposite side of the wheel:
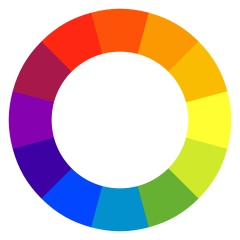
In turn, each color of light corresponds to a different frequency/wavelength of light. The absorbance of light at different wavelengths can be plotted in an absorption spectrum, which can be used to help characterize a compound:
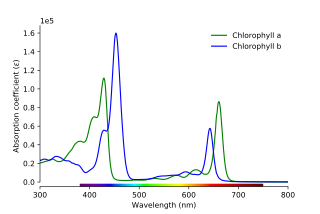
Procedure
- This is a multi-week experiment. Your notebook will be graded when you finish the HPLC part of the experiment.
- It will be conducted in pairs.
- Before conducting this experiment, please review the directions on vacuum filtration and using the SpectroVis Plus in Using Laboratory Equipment.
- As this is a research project and particularly since it’s the first time we’ve tried this, the procedure is prone to revisions and change.
Week 1: Extraction and Thin-Layer Chromatography
This week, you will
- extract the pigments using organic solvents and hot water.
- analyze the extracted pigments using TLC.
Required Special Materials and Chemicals
- Mortar and pestle
- Leaves
- sand (a pinch)
- Sodium sulfate (15 g)
- saturated sodium chloride solution (2 mL – use from provided bottle)
- acetone (15 mL)
- hexane (15 mL)
- TLC plates (2)
- 110 mm diameter filter paper (2)
- forceps (1)
Extraction
- Observe and record the appearance and species of the leaves. Take a photograph of the leaves.
- Weigh out 1.2 g of fresh leaves. Record the species and mass to the nearest 0.001 g, as well as a description of the leaves.
- Tear or cut the leaves into small pieces and place them in a mortar with a pinch of sand (to assist with grinding up the leaves). Add 6 mL of acetone and 6 mL of hexane and grind the mixture for 3–5 minutes in the fume hood. If little liquid is left in the mortar (<3 mL), add another 3-6 mL of each solvent and grind for another minute. You’re looking for at least 3 mL of a dark green liquid in your mortar.
- Filter the green solution using gravity filtration, into a clean 50 mL graduated cylinder then transfer it to a clean test tube. Note: do NOT filter directly into the test tube as it is likely that the test tube will break if you do.
- Wash the solution in the test tube:
- Add 2 mL of saturated aqueous sodium chloride solution to the test tube.
- Use a pipette and pipette the mixture up and down until it is well mixed.
- Place the test tube in the test tube rack and allow the mixture to separate.
Create a filtering pipet using a short (5″) disposable Pasteur pipet and cotton plug by tearing off a VERY SMALL (as small as possible) piece of a cotton ball, putting it into the top of the 5″ (short) Pasteur pipet and gently pushing it to the bottom of the pipet with a 2nd, longer (9″) pipet (the point of the cotton plug is just to prevent the sodium sulfate from falling out of the pipet, so don’t use too much cotton and don’t pack the cotton down too tightly). Fill the filtering pipet half-way with solid sodium sulfate using a weigh paper funnel. The final traces of water are removed by treating the organic solution with sodium sulfate as a drying agent.
- Filter the green organic layer through the filtering pipet into a screw cap vial by using a transfer pipet to move the green top layer from the test tube into the top of the filtering pipet and letting the liquid flow through the sodium sulfate and into the screw cap vial. Avoid adding any of the aqueous layer to the filtering pipet.
- Spot and run two TLC plates with your filtered green organic solution (see below for details).
- Loosely cap and label (initials, date, leaf pigment in hexane/acetone) the vial and store the vial in a small beaker (so it can’t tip over) in your drawer for next week. Be sure to NOT have the cap screwed on tightly, otherwise the solvent won’t fully evaporate. The solvent will evaporate away during this time leaving the pigment as residue. We will redissolve this residue next week.
Thin Layer Chromatography
Create the developing chambers:
- Place a 110 mm diameter filter paper into a 400 mL beaker such that it is pressed up against the side of the beaker and touching the bottom of the beaker (you will need to fold the filter paper over a bit to create a flat bottom in order to get it to fit into the beaker). You will need 2 beakers, 1 for hexane and 1 for acetone (I recommend labeling each beaker with tape so you don’t forget which beaker contains which solvent).
- Create a lid for each beaker out of aluminum foil by covering the top of the beaker with a square of aluminum foil and shaping it to the top of the beaker to seal it as tightly as possible.
- Add ~5 mL (measure using a transfer pipet) of hexane to one beaker and ~5 mL of acetone to the other beaker and IMMEDIATELY cover the top of the beaker with the aluminum foil lid (seal as tightly as possible).
- If you draw up and expel the eluent in your transfer pipet several times before transferring it to the beaker, the solvent won’t drip out of the pipet as much.
- Always hold pipets up and down (perpendicular to the floor), never sideways (parallel to the floor) as holding it sideways will cause the liquid to spill out.
- Gently swirl the beaker so that the solvent completely wets the filter paper (this will ensure that the atmosphere in the developing chamber is saturated with the solvent vapor, which helps ensure reproducible results).
- At this point, there should be a layer of solvent in that bottom of the beaker that’s ~0.5 cm thick. If it is significantly less than this, add a little more solvent.
Spot your TLC plates with your samples:
- Take a TLC plate from the box (there is often a clear plastic covering on the top of each stack of TLC plates, this isn’t a TLC plate; TLC plates are clear shiny plastic on one side and matte white silica gel, which looks a lot like plaster, on the other side).
- Never touch the side of the TLC plate that has the silica gel with gloves. It can damage the silica gel and make the TLC plate unusable. Only touch the back (the plastic side) or the sides of the TLC plate.
- Never bend a TLC plate as this will cause the silica gel to flake off and make the TLC plate unusable.
- Using a NON-MECHANICAL PENCIL (the ink in pens will dissolve in organic solvents, and mechanical pencils will scratch off the silica gel), label the top of each of your TLC plates with your initials and the date.
- Label the bottom of your TLC plate with the eluent you will use to develop that plate (meaning one TLC plate should be labeled as hexane and the other one as acetone).
- Make 2 small horizontal lines 1 cm from the bottom of the TLC plate on each edge of the TLC plate (see fig. 1).
- Make 3 small (~1 mm) vertical tic marks 1 cm from the bottom of the TLC plate and ~0.5 cm away from the sides of the TLC plate and each other and label these tic marks “1”, “2”, and “3”.
- Dip a capillary tube into your filtered green organic solution. Capillary action will automatically suck up a small amount of the solution into the capillary tube as soon as the tip of the capillary tube hits the surface of the liquid.
- Don’t put your finger over the top opening of the capillary tube, doing so will prevent the liquid from going in and out of the tube.
- Lightly press the tip of the capillary tube onto each tic mark on the TLC plate until you see a dark spot appear. This dark spot is the liquid being pulled out of the capillary tube and onto the TLC plate. The longer you hold the capillary tube on the TLC plate, the bigger this spot will get. You want the spot to be ~1 mm in diameter (if your tic mark is ~1 mm, you can use your tic mark to determine when you can lift up on the capillary tube). Always err on the side of adding too little since you can always add more.
- You can usually make 2-3 spots from a single dip into your organic solution, but if you’re concerned you won’t have enough solution left in your capillary tube to make your next spot (keep an eye on where the solvent level is in your capillary tube before and after making each spot), just dip the capillary tube back into your organic layer.
- After the organic solvent has evaporated from the spots on your TLC plate (when the spot is no longer as dark; this should only take a few seconds), create a 2nd spot of the same size on top of the spots in the “2” and “3” lane. This is known as double-spotting and is done if you’re concerned that adding a single spot won’t add enough of the compounds of desire to see after running.
- Add a 3rd spot to the “3” lane after the organic solvent has evaporated again. This means that you should now have 3 lanes, each with a single dot over the tic mark. The “1” lane is single-spotted, the “2” lane is double-spotted, and the “3” lane is triple-spotted. This way it is more likely that one of the lanes will have the right amount of the mixture to be able to see each compound as a distinct spot.
- Repeat this process of creating a single, double, and triple spot on the 2nd TLC plate.
Run your TLC plate:
- Place your prepared TLC plate into the appropriate developing chamber such that the bottom of the TLC plate is in the solvent and the top of the TLC plate is resting against the side of the chamber at a ~45-degree angle and immediately replace the aluminum foil lid on top of the beaker (seal as tightly as possible).
- Make sure that the TLC plate is oriented such that you can easily watch the solvent travel up the plate (places with solvent will be darker).
- Make sure the solvent level is well below where you spotted your compounds, otherwise they can dissolve into the solvent. If this happens, you will need to make a new eluent chamber and a new TLC plate.
- Let the TLC plate run until the solvent gets ~1 cm from the top of the plate or for 10 minutes, whichever comes first.
- I’ve found that 10 minutes usually comes first.
- During these 10 minutes, you should be doing something productive, such as cleaning up, so make sure you pay close attention to each TLC plate and exactly when each plate started running so that you know when to stop each plate.
- Using a pair of forceps, grab the top of the TLC plate and remove it from the developing chamber.
- IMMEDIATELY draw a line showing where the solvent made it on the TLC plate.
- The solvent will start evaporating immediately, so the longer you wait to draw this line, the less accurate your results will be.
- The solvent might “smile”, meaning that the solvent closer to the edges ran slightly faster than the solvent in the middle. If this happens, the line you draw should reflect this.
- Once all of the solvent has evaporated from your TLC plate (i.e., it’s no longer dark anywhere), make a sketch of the TLC plate in your lab notebook (including a ruler) and take a picture of the TLC plate (including a ruler).
Week 2: Column Chromatography
This week, you will
- separate the pigments using microscale column chromatography.
- measure the absorption spectrum of each of the pigments.
Required Special Materials and Chemicals
- acetone (15 mL)
- hexane (15 mL)
- silica gel (about 1-2 cm3)
- sand (a pinch)
Column Chromatography
We will now separate out the different pigments in the pigment sample you extracted above, to isolate different pigments and characterize their absorption spectra.
- Once you start the elution process, it cannot be stopped. You must go to completion. Be sure to read all directions for this part before proceeding. Everything must be staged so that the process can occur seamlessly.
- One of the most important rules is that columns must not be allowed to go dry. A continual flow of solvent is therefore necessary to ensure that the resin remains wet and intact.
- As glass cuvettes are fragile, be sure to handle these carefully. These will need to be shared between all members of the class.
- The first fraction eluted is beta-carotene, and the second is chlorophyll (you may be able to separate those further, but likely not).
- If necessary, add ~0.4 mL of hexane (measure using a transfer pipet) to your vial and gently mix to reconstitute (redissolve) your pigments.
- Prepare a column:
- Get a small piece of cotton or glass wool and wedge this into the narrow end of a short Pasteur pipet the same way you did last week for the filtering pipet. This should be relatively loose so liquid can get through but solid cannot.
- Scoop silica gel carefully into the column using a weigh paper funnel and make sure the column is collapsed so the powder falls to the bottom. Fill the pipet until it is about 2/3 full.
- Gently tap the side of the pipet for one minute to pack the silica.
- Add 0.5 cm of sand to the top of the pipet.
- Place the pipet column in a pipet clamp and secure it to the ring stand.
- Prepare 2 clean, small test tubes to collect the column fractions and a 100 mL beaker for waste. Label the TOP of the test tubes with your initials, the date and what fraction you will collect in that test tube (fraction 1 or fraction 2).
- Obtain 15 mL of acetone and 15 mL of hexane in separate beakers (keep these beakers covered as much as possible to minimize evaporation).
- Before elution, the column must be equilibrated (i.e. made to have similar solvent conditions as the mobile phase). To do this, place the waste beaker under the pipet column and elute 4-6 mL of hexane through the column. Note: If air bubbles or cracks appear in the column, discard and repeat Step 2.
- Allow the solvent to drain naturally. Once the solvent has drained to just above the silica (just below the sand), pipet about 75% of the green pigment solution from the last week (no more than ~0.5 mL) evenly onto the column. Store the remainder of the pigment solution in your drawer as you did last week (with the lid of the vial somewhat loose and the vial in a small beaker) to let the solvent evaporate again, leaving the pigment as residue. We will redissolve this residue next week.
- Note: It is important here that while there is a little mobile phase left, there isn’t too much hexane left on top of the column. If there is too much then this would dilute the pigment solution and “blur” the separation between the pigments.
- Allow the solution to adsorb onto the silica by letting it run into the column. Once the solution has adsorbed into the column (moved just below the sand) but before the silica is exposed to air, continue adding hexane continuously. Observe and record the migration of the bands that appear. Continue collecting all clear liquids in a waste beaker.
- A yellow band will appear and begin to separate from the green band. Continue adding hexane until you finish collecting the yellow fraction in a clean test tube.
- Once you have collected the yellow fraction, change the solvent by adding acetone instead of hexane. Continue adding the solvent and collect the clear portion in the waste beaker.
- The green band should be moving down the column. Collect this fraction in a clean test tube.
Note: Not all of the pigments will be removed from the column.
- Measure the absorption spectrum of each of the fractions using a small test tube (instead of a cuvette).
Week 3/4: High Performance Liquid Chromatography (HPLC)
You will analyze the final leaf extract using HPLC. The column we use is a C-8 column with 5 mm diameter (4.6 mm internal diameter x 100 mm length). The solvents used are:
- Solvent A: 80:20 methanol:water
- Solvent B: 95:10 acetonitrile:water
These solvents are a mixture of methanol (CH3OH) and water or acetonitrile (CH3CN) and water. A uniform solvent is used for elution. We will measure the absorbance of the sample at 440 nm and use this to generate the chromatograph.
The method is as follows with a flow rate of 1 mL/min
- 0 min-5 min: linear gradient from 100% solvent A to 100% solvent B
- 5 min-20 min: 100% solvent B
Your instructor will provide more information about the method closer to the time of the experiment.
- Your instructor will do this step: Load the method directed by your instructor and allow the system to equilibrate.
- The system needs to be equilibrated so there’s nothing in the column before you start another round. The chromatogram should be blank for a couple of minutes before you start.
- Look around and make sure there are no leaks in the system.
- Clean the syringe by drawing up 50:50 hexane:acetone mixture into the syringe and ejecting it into waste.
- Make sure the manual injector is in the upward direction.
- Get the sample vial from last week and redissolve in a small amount of 50:50 hexane:acetone mixture.
- Gently draw up a little more than 20 μL of the liquid with the special syringe, making sure there are no air bubbles. If there are air bubbles, eject all the liquid and try again more slowly, making sure that the tip of the needle is always submerged in the liquid. Inject the sample into the injector.
- When the injection is completed, push the injector knob downwards so that the sample is injected. Make sure no air bubbles are injected it into the system. The HPLC system will record the starting time.
- Clean the syringe by drawing up 50:50 hexane:acetone mixture into the syringe and ejecting it into waste.
- Wait until the method is complete. Make a PDF of the report and email the PDF to yourself.
Sharing of Data
You will post the PDF along with information about the leaves (species and overall appearance) onto Canvas by midnight the day you complete your lab report.
Data Analysis
- Locate spectra of different plant pigments and compare against the spectra for the pigments you extracted in the first week. Could you identify these pigments?
- For the HPLC experiment, refer to Wright, S. W. et al, Improved HPLC method for the analysis of chlorophylls and carotenoids from marine phytoplankton. Mar. Ecol. Prog. Ser. 1991, 77, 183-196 and compare the peaks observed to yours. Note that since the method is slightly different I would not expect your spectra to look exactly the same as the spectra in this paper.
Acknowledgment:
The extraction and column chromatography procedure is based on a protocol used previously for extracting pigments from spinach leaves for organic chemistry.
- For further details, please read Hoefnagels, Biology: The Essentials, 4th Ed, Ch. 5.2 (Photosynthetic Pigments) or OpenStax Biology 2e, Ch. 8.2. ↵

