10 Analysis of Acids and Bases by Titration
Purpose
Determine the concentration of a sample of vinegar using acid-base titration methods.
Expected Learning Outcomes
- Describe the chemistry of acid-base neutralization reactions.
- Solve stoichiometry problems in the solution phase.
- Prepare standard acid solutions and use this to standardize a basic solution.
- Determine the concentration of a solution of vinegar and compare this with the stated concentration.
Theoretical Background
Textbook Reference
- Tro, Chemistry: Structures and Properties, 2nd Ed, Ch. 8.7.
- Tro, Chemistry: A Molecular Approach, 5th Ed, Ch. 5.7.
Acid-Base Neutralization Reactions
There are a number of different definitions of acids and bases present. However, for most purposes, the Brønsted-Lowry definition of acids and bases are the most useful:
- Acid: a proton (H+) donor. Common examples include hydrochloric (muriatic) acid, citric acid, and ascorbic acid (Vitamin C).
- Base: a proton acceptor. Among the best known of these substances are hydroxides, such as sodium hydroxide; however, other bases exist. Examples of these include sodium bicarbonate (baking soda) and sodium hypochlorite (bleach).
The most commonly observed type of acid-base reaction – and the type of acid-base reaction that we will study in this experiment – is an acid-base neutralization reaction:
acid + base → water + salt
where the identity of the salt can be determined by taking the cation from the base and taking the anion from the acid.
Example
If I react sodium hydroxide with hydrochloric acid, then the balanced chemical equation is
[latex]\ce{HCl}(aq) + \ce{NaOH} (aq) \to \ce{H2O}(l) + \ce{NaCl}(aq)[/latex]
and the salt produced is sodium chloride (NaCl) - the Na+ comes from the sodium hydroxide, while Cl- comes from the hydrochloric acid.
In this experiment, you will study this reaction in a titration experiment.
Titrations
A titration experiment is one where one attempts to determine the concentration of a sample solution by reacting two samples in such a way that the exact volume of the two reactants are known. If the concentration of one of the two reactant solutions is known exactly, then one can determine the concentration of the second solution accurately.
As illustrated below, a measured volume of the analyte - typically the acid - is placed in the Erlenmeyer flask at the bottom along with an indicator. The indicator is a compound that will change color when the reaction has completed to the end point.[1] A volumetric pipet is typically used to measure out the exact amount of analyte needed.
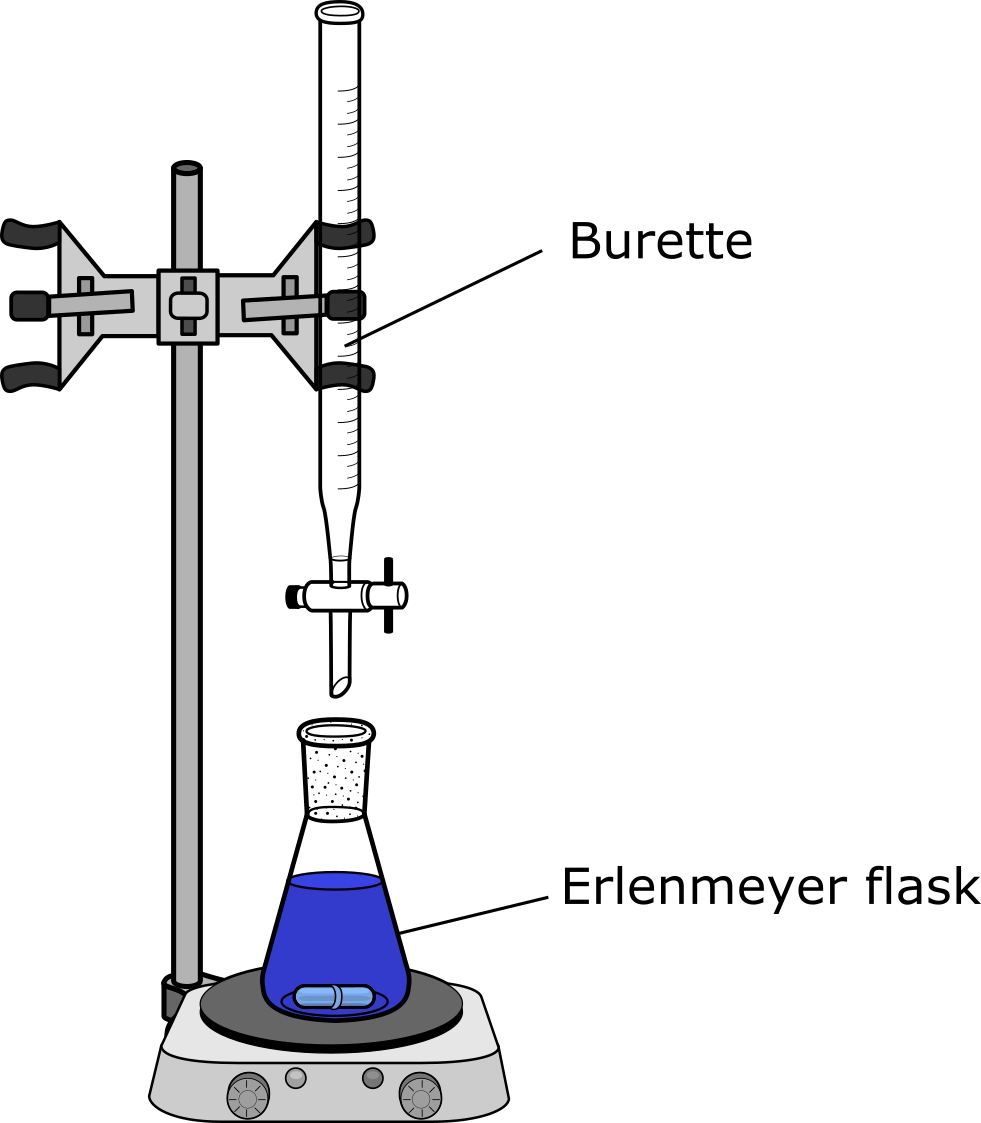
A buret is used to deliver the titrant (typically the base in acid-base titrations). The titrant is added in dropwise such that the volume of titrant required to change the color of the indicator can be recorded to the nearest 0.01 mL. Therefore, we can determine the exact (or as close to exact as we can) volume of titrant required to react with the analyte.
In this experiment, you will use the acid (potassium hydrogen phthalate in the first part, and vinegar in the second part) as an analyte, and sodium hydroxide (a base) will be used as the titrant. You will use phenolphthalein as the indicator.
About This Experiment
The goal of this experiment is to determine the concentration of white vinegar, which is essentially a solution of acetic acid ([latex]\ce{CH3COOH}[/latex]). To do this, we need a standard solution to use as a starting point. A standard solution is a solution that can be used to determine the concentration of another solution.
Standardization of Sodium Hydroxide
While we can try and prepare a solution of sodium hydroxide of a given concentration using the standard method for preparing solutions, the concentration of this solution is actually not accurately known. Sodium hydroxide cannot easily be used as a primary standard because it tends to be extremely hygroscopic (absorbs water from the air) and therefore the mass of sodium hydroxide used is not an accurate starting point for determining the concentration of base used.
For this reason, we use KHP as a primary standard with which the concentration of the sodium hydroxide solution can be determined accurately (and therefore act as a secondary standard). KHP is a monoprotic acid, and undergoes the neutralization reaction
[latex]\underbrace{\ce{HC8H4KO4}}_{\mbox{KHP}} (aq) + \ce{NaOH}(aq) \to \ce{H2O}(l) + \ce{NaKC8H4O4} (aq)[/latex]
It is prepared with a substance that is either pure or of known purity, stable, and easily handled during the preparation. For the standardization of a base a standard solution of a solid acid such as potassium hydrogen phthalate (abbreviation KHP; the formula is [latex]\ce{HC8H4KO4}[/latex] (MW = 204.2 g/mol; monoprotic)) is prepared.
- A common error made by students is to use KHP as the formula for potassium hydrogen phthalate and use that to find the molar mass.
- Potassium hydrogen phthalate (KHP) is in fact somewhat hygroscopic. When water is absorbed, it will clump together and not be free to flow. When this happens, it needs to be dried in an oven for about an hour. For the purpose of this class, please find another container of KHP instead.
From the information obtained the concentration of the provided sodium hydroxide solution can be determined.
The end-point - which is when the analyte is completely reacted with the titrant - is determined using phenolphthalein as an indicator. When the end-point of the titration is reached, the color of the solution changes from colorless to pink.
Determining the Concentration of Acetic Acid in Vinegar
Given the concentration of the sodium hydroxide, one can determine the concentration of acetic acid in vinegar. The acetic acid is neutralized by the sodium hydroxide as follows:
[latex]\ce{CH3COOH} (aq) + \ce{NaOH} (aq) \to \ce{H2O}(l) + \ce{NaCH3COO} (aq)[/latex]
Again, you will use phenolphthalein as the indicator for this titration.
Procedures
Hints and Safety Rules
- This experiment will be completed in pairs, but to get full-credit for your in-lab performance, both partners must do AT LEAST 3 careful titrations and at least 1 quick titration.
- Review Using Standard Laboratory Equipment to ensure that you know the procedures for using a buret, volumetric pipet and volumetric flask.
- It is important to remember in this experiment that the bottom of the meniscus is where the liquid level should be measured. All volumetric pipets and flasks are accurate to four significant figures, while the buret should be read to the hundredths place. Remember that the buret is graduated with 0 mL at the top and 50 mL at the bottom. Pipets will have four significant figures.
- It is important that you mix all the solutions in the volumetric flasks as well as possible. In my experience, if you do not mix up the flask completely, you will find that your results are imprecise.
- Be sure to use deionized water for all purposes except rough cleaning of glassware.
- Do not blow out the last drop of solution from the tip of a pipet into the Erlenmeyer flask – that would add too much solution.
- Your first titration of a NEW sample (i.e. a sample you have never titrated before) should always be a quick and dirty titration where you quickly add the titrant (the solution in the buret) to see approximately where the end point of this titration will be, understanding that you will go significantly past the true end point, thus the data you get from this quick and dirty titration will be inaccurate and should NOT be used to calculate the concentration of your titrate (the solution in the Erlenmeyer flask). This will allow you to quickly add most of the tirant needed in your real trials and thus only need to carefully add the last mL or two drop by drop to get to the true end point.
- While part of your grade for this lab will be based on the accuracy and precision of your titrations, it is important that you don't spend too much time trying to be perfect with them as doing so will cause you to not finish all of the titrations you are supposed to do, which will affect your grade far more than having slightly worse accuracy and precision to your data.
Chemicals Needed
Pour out the following amounts of chemicals into your own glassware. Be sure to label the glassware appropriately before pouring the chemicals into said glassware:
- Vinegar: 15 mL
- Sodium hydroxide solution: 150 mL
- Potassium hydrogen phthalate (KHP): 4 g. You will measure this out directly from a weigh boat provided by your instructor near the balance.
- Phenolphthalein: take drops directly from bottle as needed.
Preparation of Standardized Solution of Potassium Hydrogen Phthalate (KHP)

You will prepare a 100 mL solution of KHP using a volumetric flask. There is a graduation mark on the narrow neck of the flask. Remember that the bottom of the meniscus should match exactly that line. Be very careful; you will have to re-make the solution if you overshoot in making the solution.
- Carefully weigh a clean, dry 50 or 100 mL beaker as accurately as possible.
- Place between 2.9 g and 3.2 g potassium hydrogen phthalate (KHP) in the beaker and weigh accurately (add the KHP directly into the beaker, don't use a weigh paper).
- Transfer the weighed crystals to a clean 100 mL volumetric flask, being careful to prevent loss of the crystals. To ensure every bit of KHP is transferred, rinse the beaker and the funnel with three small portions of deionized water into the volumetric flask.
- Fill the volumetric flask half-full of deionized water, stopper it (parafilm will work if no stoppers are available), and swirl to dissolve the crystals. When all solid is dissolved, pour deionized water into the volumetric flask until the level is ~1 cm below the mark, then use a transfer pipet to add deionized water dropwise until the bottom of the meniscus is at the same level as the mark, then mix thoroughly.
Standardization of the Sodium Hydroxide Solution
You will now determine the exact concentration of the sodium hydroxide solution that is provided in the laboratory, by titrating the KHP solution prepared above using the ~0.1 M NaOH solution provided for you.
- Rinse your burets thoroughly with tap water and deionized water. A clean buret will drain freely without forming drops on the inside surface. Insure that the stopcock functions properly; it must turn freely and must not leak when turned off.
- Rinse the buret with 5 mL portions of the NaOH solution, by pouring the solution into the barrel and running it through the stopcock and tip of the buret. Discard the rinse solution.
- Fill the buret approximately to the 10.00 mL mark with the NaOH solution. Place the buret cap on the buret to reduce the rate of reaction between sodium hydroxide and atmospheric carbon dioxide.
- Using a 10 mL volumetric pipet, transfer 10.00 mL of the KHP solution into a clean, rinsed 250-ml Erlenmeyer flask. Touch off the pipet tip if necessary and rinse the inside walls of the flask with a minimum amount of deionized water.
- Add enough deionized water such that the solution covers the bottom of the flask. Add 2 drops of phenolphthalein indicator, and swirl the Erlenmeyer flask to mix the solution evenly.
- Position the Erlenmeyer flask under the NaOH buret. Place a sheet of white paper under the flask to help you see the indicator more easily.
- Record the initial volume in the buret.
- Begin titrating the KHP with NaOH by opening the stopcock of the NaOH buret almost all the way. Immediately begin stirring the solution by swirling the flask.
- For your initial quick and dirty titration, continue quickly adding the NaOH until the KHP solution first turns entirely pink. Then close the stopcock, read the volume on the buret and calculate how much volume of NaOH solution was added to the KHP (this should be somewhere between 10 and 20 mL).
- Repeat steps 4-8. When doing so, you should try and add the sodium hydroxide relatively quickly until you are about 2-3 mL from the expected end point from the previous attempt, then add small squirts of the sodium hydroxide; this will help speed up the titration. You might need to add more sodium hydroxide before you begin the titration if you're unsure you will have sufficient sodium hydroxide to complete the titration (i.e., if you need to add so much NaOH that the meniscus would be below the 50 mL mark at the end point). As you approach the end point of the titration, the color of the indicator in the flask will appear in parts of the unmixed NaOH solution and will persist for several seconds. Be sure to swirl the flask to ensure that the color of the indicator does not persist. At this point, decrease the rate of addition until you are adding single drops and thoroughly mixing the solution after each drip is added.
- Make sure you can initially see a pink color in the center of the flask when you add your NaOH (but it should disappear after swirling it). If you don't see an initial pink color, you probably forgot to add the phenolphthalein indicator.
- If it takes you >10 minutes to approach the endpoint of the titration, something has gone wrong and you should treat this trial as another quick and dirty titration and quickly add the NaOH until you get an obvious pink color and compare the volume added to the volume added from your original quick and dirty trial to try to figure out what went wrong.
- The end point is reached when a single drop (or fraction of a drop) produces a faint pink color which does not disappear after 30 s of vigorous swirling. At this point, record the volume of sodium hydroxide in the buret. It is not desirable that the solution turns deep pink - the last drop should be able to turn the solution such that it has a faint, yet permanent, shade of pink.
- Repeat steps 10 and 11 at least two more times (although if you have less than 30 minutes remaining, you are better off skipping to step 13 so that you can complete at least 1 titration of vinegar).
- You should complete a fourth trial if the volume delivered from the first three trials are more than 0.1 mL apart. Note that a quick and dirty titration does NOT count as a trial since it is known to be inaccurate, and you should never use data you know to be inaccurate (although you should still record it in your in-lab notebook since that is a record of everything you do, regardless of whether it is accurate or not).
- When you are sure you have completed this part of the experiment, thoroughly wash and clean the volumetric flask and volumetric pipet.
At this point in the lab, you should do 1 molarity calculation (see below) for the ~0.1 M NaOH solution to make sure that you know how to do it. If you struggle with this calculation, get help from your instructor. But do NOT do all of the molarity calculations at this point as you can finish them after lab, but you can't finish the vinegar titrations after lab.
Determining the Concentration of Vinegar
Now that you have a standardized solution of sodium hydroxide, you can use this to determine the concentration of acetic acid in vinegar.
- Record the brand and appearance of the vinegar provided.
- Prepare a diluted solution of vinegar by dispensing 10.00 mL vinegar using a volumetric pipet into the 100.0 mL volumetric flask and diluting the vinegar with deionized water, being sure to mix the solution carefully.
The best way of making sure that the solution is mixed up is to fill up most of the flask (such that the large bulb is ~75% full), and the swirl the flask several times. Then, make up the volume to the mark. Seal the flask with a piece of parafilm, and invert the flask several times to make sure the sample is completely mixed. - Rinse your pipet with deionized water followed by approximately 5 mL of the diluted vinegar solution.
- Titrate the diluted vinegar solution against the 0.10 M sodium hydroxide solution following the same protocol as above.
If you have time before the end of lab, continue working on your molarity calculations so that you can get help from your instructor if you get stuck.
Waste Management
All waste solutions from this experiment can be poured down the drain with plenty of water.
Data Analysis
This laboratory experiment is rather complex to calculate and takes some time to do. Be sure to complete all calculations carefully. If you do not understand how to do these calculations, please be sure to consult your instructor or a mentor in the Math/Science Resource Center.
Determining the Concentration of Potassium Hydrogen Phthalate Solution
Potassium hydrogen phthalate has a molar mass of 204.2 g/mol. Given the mass of the potassium hydrogen phthalate present and the volume of the volumetric flask (which you will need to convert into liters), you should be able to determine the molarity of the solution using the definition
[latex]\mbox{molarity (M)} = \frac{\mbox{moles of solute}}{\mbox{liters of solution}}[/latex]
Example
If 3.224 g potassium hydrogen phthalate (KHP) was dissolved in 100.0 mL deionized water, the molarity of the KHP solution is
[latex]\begin{align*} ?\mbox{ mol KHP} &= 3.224\mbox{ g KHP} \times \frac{1\mbox{ mol KHP}}{204.2 \mbox{ g KHP}} \\ &= 0.01579\mbox{ mol} \\ ?\mbox{ L soln} &= 100.0 \mbox{ mL} \times \frac{1\mbox{ L}}{1000\mbox{ mL}} \\ &= 0.1000\mbox{ L} \\ ? \mbox{ mol/L} &= \frac{0.01579 \mbox{ mol}}{0.1000\mbox{ L}} \\ &=0.1579\mbox{ M} \end{align*}[/latex]
Determining the Concentration of the ~0.1 M Sodium Hydroxide Solution
This can be solved as a solution stoichiometry problem. To do this, the first step is to determine the number of moles of sodium hydroxide present. Based on the balanced chemical equation
[latex]\underbrace{\ce{HC8H4KO4}}_{\mbox{KHP}} (aq) + \ce{NaOH}(aq) \to \ce{H2O}(l) + \ce{NaKC8H4O4} (aq)[/latex]
one can determine - based on the volume and molarity of KHP - the number of moles of NaOH in the solution. This can then be used to find the molarity of the sodium hydroxide solution.
Example
10.00 mL of a 0.1523 M KHP solution requires 14.22 mL of NaOH solution to neutralize. The sodium hydroxide solution has the molarity
[latex]\begin{align*} ?\mbox{ mol NaOH} &= 10.00\mbox{ mL KHP}\times \frac{1\mbox{ L KHP}}{1000\mbox{ mL KHP}} \times \frac{0.1523 \mbox{ mol KHP}}{\mbox{L KHP}} \times \frac{1\mbox{ mol NaOH}}{1\mbox{ mol KHP}} \\ &= 0.001523\mbox{ mol NaOH} \\ ? \mbox{ L NaOH} &= 14.22\mbox{ mL} \times \frac{\mbox{L}}{1000 \mbox{ mL}} \\ &= 0.01422\mbox{ L}\\ ?\mbox{ M} &= \frac{0.001523\mbox{ mol}}{0.01422\mbox{ L}} \\ &= 0.1071\mbox{ M} \end{align*}[/latex]
Determining the Concentration of Vinegar
Given the molarity of the standardized base and the volume of standardized base delivered via the buret, the number of moles of acetic acid in the diluted vinegar solution can be determined based on stoichiometric calculations similar to the previous part.
The actual concentration of the vinegar can then be done using the reverse of a dilution calculation. This can be completed using the equation
[latex]M_1V_1 = M_2V_2[/latex]
Distilled vinegar is nominally 5% (w/v) acetic acid - i.e., 5 g acetic acid per 100 mL of vinegar. By converting this to molarity, you should be able to compare your results with the nominal concentration of acetic acid in vinegar.
- This is explained in CHEM-C 106. ↵

