2 Physical and Chemical Properties and Separation of a Mixture
Purpose
To observe and evaluate some changes that are observed in chemistry, and to use differences in solubility to separate different substances within a mixture.
Expected Learning Outcomes
After performing this experiment, students should be able to
- Use a Bunsen burner effectively and safely in the chemical laboratory. (LO 3)
- Identify the differences between chemical and physical changes under similar experimental conditions. (LO 3)
- Identify the differences between chemical and physical properties, and to observe some of the properties of particular pure substances. (LO 3, 4)
- Utilize the properties of pure substances to separate the components of a simple mixture. (LO 3)
- Determine the mass of different objects, and calculate the percent by mass of a substance. (LO 3)
- Use reference works to determine strategies to separate different substances. (LO 6)
Textbook Reference
Tro, Chemistry: Structures and Properties, 2nd Ed, Ch. 7.2
Introduction
Chemical and Physical Properties
Substances can be identified by different properties that they can have.
Examples
- When you heat water to about 100°C, it boils.
- A solution of copper (II) sulfate is blue.
- When you add Alka-Seltzer to some vinegar, you see bubbles form.
- If you overcook a steak, it chars and you see black bits on it.
Furthermore, substances can change from one form to another, with some different properties.
These changes and properties can be classified as physical or chemical properties. In the first part of this experiment, you will focus on classifying changes in matter as either physical changes or chemical changes.
Physical Properties and Changes
These properties are associated with the substance rather than its molecular structure – that is, without changing the identity of the substance. These properties can be observed without observing any change at the molecular level.
There are a number of important classes of physical changes that you need to know about:
- You can create mixtures by mixing two substances together into a mixture. For example, I can dissolve drink powder to water.
- Conversely, you can – by various different means such as filtering or selectively dissolving components – separate components of a mixture.
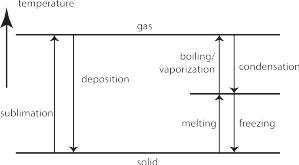
- Phase changes involve changing the state of a particular substance through heating/cooling or (less commonly) changing the pressure on a substance. There are specific terms used to describe different phase changes which you need to know. The terms for these phase changes are illustrated below and need to be learnt.
Chemical Properties and Changes
In contrast, chemical changes involve chemical reactions, where the identity of substance(s) change.
Examples
- Two or more elements can react together to form a compound.
- A compound can decompose into its constituent elements.
- One or more compounds can react to form one or more compounds.
In this case, the original substance no longer exists. Instead, new substances are formed that have distinctly different properties. For instance, cooked fish is significantly softer and less translucent than raw fish.
Distinguishing Between Physical and Chemical Changes
While the difference between physical and chemical changes may appear clear, it’s not necessarily the easiest thing to distinguish in an experiment. However, the main thing to do is to look at as many properties as possible – and not just one.
Typically, a physical change would involve changes of a relatively small range of properties – since it’s still essentially the same substance.
Examples
On the other hand, after a chemical change a new substance is formed, and therefore we expect a significant change in the substance’s properties. Therefore, if all (or nearly all) of the properties have changed, then we can reasonably conclude that a chemical change has occurred.
Examples
Separation of a Mixture
There are a number of different ways by which a mixture can be separated, and is a common problem in a chemical laboratory. It is important to separate the components of a mixture in many contexts, both in the laboratory (as you may learn in the organic chemistry laboratory) or in real life.
Examples
- Crude oil contains a mixture of different hydrocarbons, which need to be separated into different components in order for us to obtain the chemicals we need in life (e.g. different types of fuel).
- Water often needs to be purified in order to make it drinkable.
As you may know from everyday life, different substances dissolve in different liquids.
Examples
It is partly for this reason that we have all these different types of solvents and cleaning agents at home. In this experiment, we will separate two different components in a mixture: copper (II) sulfate (which has a distinctive blue color and is soluble in water) and calcium carbonate (which is white and is insoluble in water) by adding water to the mixture. One component will dissolve in water while the other will remain undissolved.
Procedure
- You will complete this experiment in pairs.
- Review the directions for using:
- a Bunsen burner (under Using Standard Laboratory Equipment)
- To heat a sample gently, you need to reduce the gas flow (using the gas tap and the needle valve at the bottom of the Bunsen burner) and place the test tube (or other heating element) in the middle of the outer cone, away from the hottest part of the flame.
- a Bunsen burner (under Using Standard Laboratory Equipment)
- In this experiment, you will mostly be making qualitative (descriptive) observations. No numerical results will be recorded; rather, you will write down what you see such as the physical state of the substance, the appearance (including consistency and color), and any other features that you observe before, during, and/or after the process. Highlight any differences that you see before and after a change was made.
- It is important to be as specific and precise as possible in your records. Recording too much in your notebook is not a problem; recording too little would be a problem. However, full sentences are not a concern.
- In many parts of this experiment, you will heat test tubes by clamping the test tube containing the sample in the metal test tube holders (found in the general use drawer), and then hold the sample in the Bunsen flame.
- In this experiment, you should use small test tubes where possible. Exact amounts of chemicals are not critical.
- Due to the small amounts of reagents required and the qualitative nature of this experiment, you will simply dispense the chemicals from the bottles provided.
- Since each part of the experiment is independent of each other, you should do this experiment in whatever order works best for you.
Physical and Chemical Changes
In this part of the experiment, you will observe a number of changes to the substances being studied. In each case, the goal is to determine:
- if it is a physical or a chemical change
- if it is a physical change, is it a phase change?
To complete the short report for this experiment after class, it will be critical that you are as specific and precise as possible when recording your observations in your lab notebook. You should record the state, appearance (including consistency and color) and any other features of the substance or change that you have observed. You need to record both the appearance after the change and report what changes were found.
Whenever you heat a test tube with a Bunsen burner, be sure to let it cool to near room temperature before putting it back into your test tube rack, otherwise it could melt the test tube rack. Also be sure to turn off the Bunsen burner when it is not in use, even if you are planning on using it soon, so that the risk of catching something on fire is minimized.
Sodium Nitrate
- Place a few crystals of sodium nitrate (NaNO3) into a small test tube. Note the color and general appearance of the substance.
- Using a Bunsen burner, gently heat the test tube (see above) until a change in the substance inside the test tube is noted. Record carefully what you observe and determine if this was a physical or chemical process.
- Allow the tube and contents to cool. What change is observed during the cooling process? Is this a physical or chemical process?
- After the tube is no longer hot, add enough water to dissolve the solid (tap to mix). If the solid doesn’t fully dissolve, mix with a stir rod. If it still doesn’t fully dissolve, add more water and mix again. Once the solid is fully dissolved, record the appearance of the solution (the liquid). Is this a physical or chemical process?
Copper(II) Nitrate
- Obtain a small number of crystals of copper(II) nitrate (Cu(NO3)2) and place them in a small test tube. Observe and record the general appearance of these crystals.
- Prepare a gentle Bunsen flame. Heat the test tube gently (by restricting gas flow and placing the test tube near the top of the outermost cone of the Bunsen flame).
Record what you observe inside the test tube. Did you observe a physical or chemical change?
- Now, increase the intensity of the Bunsen flame by increasing the gas flow. Turn off the Bunsen Burner, let cool to room temperature (don’t put in the test tube rack until it cools to close to room temperature), record your observations and determine whether it is a physical or chemical change. Explain your answer.
Iodine
- Carefully place a couple of crystals of iodine into a dry test tube. Observe the iodine crystals in the test tube.
- Heat the test tube very gently in a Bunsen flame. Describe the changes you observe. What does the substance look like while you are heating the test tube?
- After you have seen the change, turn off the Bunsen burner and allow the tube to cool to room temperature before putting it into a test tube rack. How do the crystals on the sides of the tube compare with the original iodine in composition and properties?
Based on your observations, determine if you observed physical or chemical changes, and if you observed a phase change, determine what this phase change is called (use this figure for the terms).
Iron(III) Chloride
- Half-fill a small test tube with water.
- Add 2-3 drops of iron(III) chloride (FeCl3) solution solution into the test tube. What does the solution look like before you add it to the water? What does it look like after adding it to the water?
- Add 2-3 drops of ammonium thiocyanate (NH4SCN) solution to the test tube with the water and iron(III) chloride (FeCl3). Record the appearance of the ammonium thiocyanate (NH4SCN) solution before adding it to the test tube and what changes you observe after adding it to the test tube, and determine whether you have observed a physical or a chemical change.
Sodium Carbonate
- Place ~0.5 mL (half way up a plastic transfer pipet) of sodium carbonate (Na2CO3) solution into a test tube and record its appearance.
- Add a few drops of 3 M hydrochloric acid (HCl) into the test tube. If no effect is observed, add a few more drops. Record any observations you make, including what the 3 M hydrochloric acid solution looks like before adding it to the test tube and what the liquid in the test tube looks like while the effect is happening.
- Wait until you observe no more changes to the liquid in the test tube and then observe what it looks like after the effect is over.
Separation of a Mixture
In this section, key aspects include:
- The color of the component/mixture being observed.
- For solids, the amount of the substance observed.
- Add to a 4″ test tube a small amount (about a teaspoonful) of the provided mixture of copper (II) sulfate and calcium carbonate. Note the appearance of this mixture. (You do NOT need to mix the solids together for this part, it is provided pre-mixed.)
- Add about 5 mL of deionized water to the test tube and shake well. Describe what you observe at this point.
- By pouring or by using a plastic transfer pipet, transfer the liquid from the test tube into an evaporating dish, being careful not to carry over any of the solid. Describe the solid residue and the liquid phase that are found at this point.
- Place the evaporating dish on a hot plate and heat gently (about 300°C). Allow most of the water in the mixture to evaporate off and describe what you observe in the evaporating dish.
Waste Management
Iodine residue in the test tube can be rinsed out with a few mLs of ethanol and rinsed down the drain with lots of water.
Dispose of all other waste in specified beakers in the fume hoods.
Acknowledgment
This experiment is based on experiments developed at Fort Hays State University for Chemistry 105.

