68 Carbon dioxide transport
Learning Objectives
After reading this section you should be able to-
- Describe the ways in which carbon dioxide is transported in blood and explain the relative importance of each to total carbon dioxide transport.
- State the reversible chemical equation for the reaction of carbon dioxide and water to carbonic acid and then to hydrogen ion and bicarbonate ion.
- Explain the relationship between pH and hydrogen ion concentration.
- Predict how changing the partial pressure of carbon dioxide will affect the pH and the concentration of bicarbonate ions in the plasma.
- Predict how changing the pH or the concentration of bicarbonate ions will affect the partial pressure of carbon dioxide in the plasma.
- State the reversible chemical equation for carbon dioxide binding to deoxyhemoglobin.
- Explain the role of each of the following in carbon dioxide transport: carbonic anhydrase, hydrogen ions binding to hemoglobin, the chloride shift, and oxygen-hemoglobin saturation level.
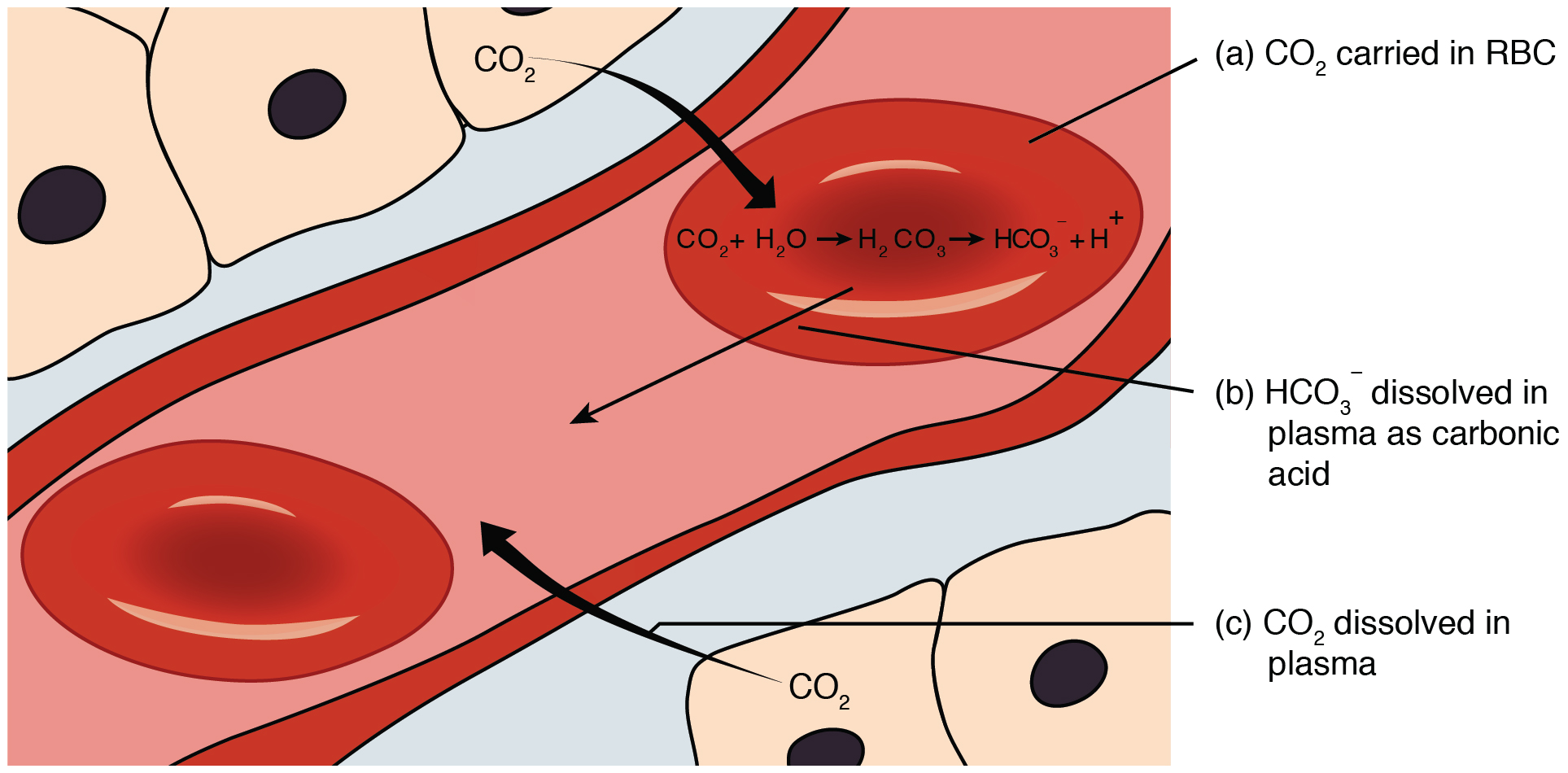
Carbon dioxide is transported by three major mechanisms. The first mechanism of carbon dioxide transport is by blood plasma, as some carbon dioxide molecules dissolve in the blood. The second mechanism is transport in the form of bicarbonate (HCO3–), which also dissolves in plasma. The third mechanism of carbon dioxide transport is similar to the transport of oxygen by erythrocytes (Figure 69.1).
Dissolved Carbon Dioxide
Although carbon dioxide is not considered to be highly soluble in blood, a small fraction—about 7 to 10 percent—of the carbon dioxide that diffuses into the blood from the tissues dissolves in plasma. The dissolved carbon dioxide then travels in the bloodstream and when the blood reaches the pulmonary capillaries, the dissolved carbon dioxide diffuses across the respiratory membrane into the alveoli, where it is then exhaled during pulmonary ventilation.
Bicarbonate Buffer
A large fraction—about 70 percent—of the carbon dioxide molecules that diffuse into the blood is transported to the lungs as bicarbonate. Most bicarbonate is produced in erythrocytes after carbon dioxide diffuses into the capillaries, and subsequently into red blood cells. Carbonic anhydrase (CA) causes carbon dioxide and water to form carbonic acid (H2CO3), which dissociates into two ions: bicarbonate (HCO3–) and hydrogen (H+). The following formula depicts this reaction:
After carbon dioxide combines with water inside red blood cells, it forms carbonic acid. This carbonic acid then breaks down into bicarbonate ions (HCO3–) and hydrogen ions (H+). Now, to maintain a balance of charges inside the red blood cells, chloride ions (Cl–) move into the cells, effectively swapping places with the bicarbonate ions. This exchange, known as the chloride shift, helps keep the electrical balance within the red blood cells, allowing them to transport carbon dioxide efficiently.
At the pulmonary capillaries, the chemical reaction that produced bicarbonate (shown above) is reversed, and carbon dioxide and water are the products. Much of the bicarbonate in the plasma re-enters the erythrocytes in exchange for chloride ions. Hydrogen ions and bicarbonate ions join to form carbonic acid, which is converted into carbon dioxide and water by carbonic anhydrase. Carbon dioxide diffuses out of the erythrocytes and into the plasma, where it can further diffuse across the respiratory membrane into the alveoli to be exhaled during pulmonary ventilation.
Carbaminohemoglobin
About 20 percent of carbon dioxide is bound by hemoglobin and is transported to the lungs. Carbon dioxide does not bind to iron as oxygen does; instead, carbon dioxide binds amino acid moieties on the globin portions of hemoglobin to form carbaminohemoglobin, which forms when hemoglobin and carbon dioxide bind. When hemoglobin is not transporting oxygen, it tends to have a bluish-purple tone to it, creating the darker maroon color typical of deoxygenated blood. The following formula depicts this reversible reaction:
Similar to the transport of oxygen by heme, the binding and dissociation of carbon dioxide to and from hemoglobin is dependent on the partial pressure of carbon dioxide. Because carbon dioxide is released from the lungs, blood that leaves the lungs and reaches body tissues has a lower partial pressure of carbon dioxide than is found in the tissues. As a result, carbon dioxide leaves the tissues because of its higher partial pressure, enters the blood, and then moves into red blood cells, binding to hemoglobin. In contrast, in the pulmonary capillaries, the partial pressure of carbon dioxide is high compared to within the alveoli. As a result, carbon dioxide dissociates readily from hemoglobin and diffuses across the respiratory membrane into the air.
In addition to the partial pressure of carbon dioxide, the oxygen saturation of hemoglobin and the partial pressure of oxygen in the blood also influence the affinity of hemoglobin for carbon dioxide. This interplay is known as the Haldane effect: deoxygenated hemoglobin (Hb) binds CO₂ much more readily than oxygenated Hb. In other words, as Hb releases O₂ in the tissues, its affinity for CO₂ increases—promoting CO₂ uptake. Conversely, when Hb picks up O₂ in the lungs, its affinity for CO₂ drops, facilitating CO₂ unloading into the alveoli.
Hemoglobin that is saturated with oxygen does not readily bind carbon dioxide. However, when oxygen is not bound to heme and the partial pressure of oxygen is low, hemoglobin readily binds to carbon dioxide.
Relationship Between pH and Hydrogen Ion Concentration
The pH of a solution is a measure of its hydrogen ion (H+) concentration; the two are inversely related. A lower pH indicates a higher concentration of hydrogen ions, whereas a higher pH indicates a lower concentration of hydrogen ions.
Effect of Partial Pressure of Carbon Dioxide on pH and Bicarbonate Ions
Changing the partial pressure of carbon dioxide affects the pH and the concentration of bicarbonate ions in the plasma. An increase in the partial pressure of carbon dioxide leads to more carbonic acid formation, which dissociates into hydrogen ions and bicarbonate ions, lowering the pH. Conversely, a decrease in the partial pressure of carbon dioxide raises the pH by reducing the concentration of hydrogen ions.
Effect of pH and Bicarbonate Ion Concentration on Partial Pressure of Carbon Dioxide
Changing the pH or the concentration of bicarbonate ions will affect the partial pressure of carbon dioxide. Lowering the pH (increasing hydrogen ion concentration) will shift the equilibrium toward producing more carbon dioxide, raising its partial pressure. Increasing the concentration of bicarbonate ions will also shift the equilibrium toward producing more carbon dioxide.
Adapted from Anatomy & Physiology by Lindsay M. Biga et al, shared under a Creative Commons Attribution-ShareAlike 4.0 International License, chapter 22
enzyme responsible for catalyzing carbon dioxide into carbonic acid
the form of hemoglobin that is bound to carbon dioxide
the phenomenon describing the relationship between the partial pressure of oxygen and the affinity of hemoglobin for carbon dioxide; deoxygenated hemoglobin has a higher affinity towards carbon dioxide than oxygenated hemoglobin

