62 Mechanisms of pulmonary ventilation
Learning Objectives
After reading this section, you should be able to-
-
Explain the inverse relationship between gas pressure and volume of the gas (i.e., Boyle’s Law) and apply this relationship to explain airflow during inspiration and expiration.
-
Explain how pulmonary ventilation is affected by bronchiolar smooth muscle contractions (bronchoconstriction), lung and thoracic wall compliance, and pulmonary surfactant and alveolar surface tension.
-
Describe the forces that tend to collapse the lungs and those that normally oppose or prevent collapse (e.g., elastic recoil of the lung versus sub-atmospheric intrapleural pressure)
Pressure Relationships
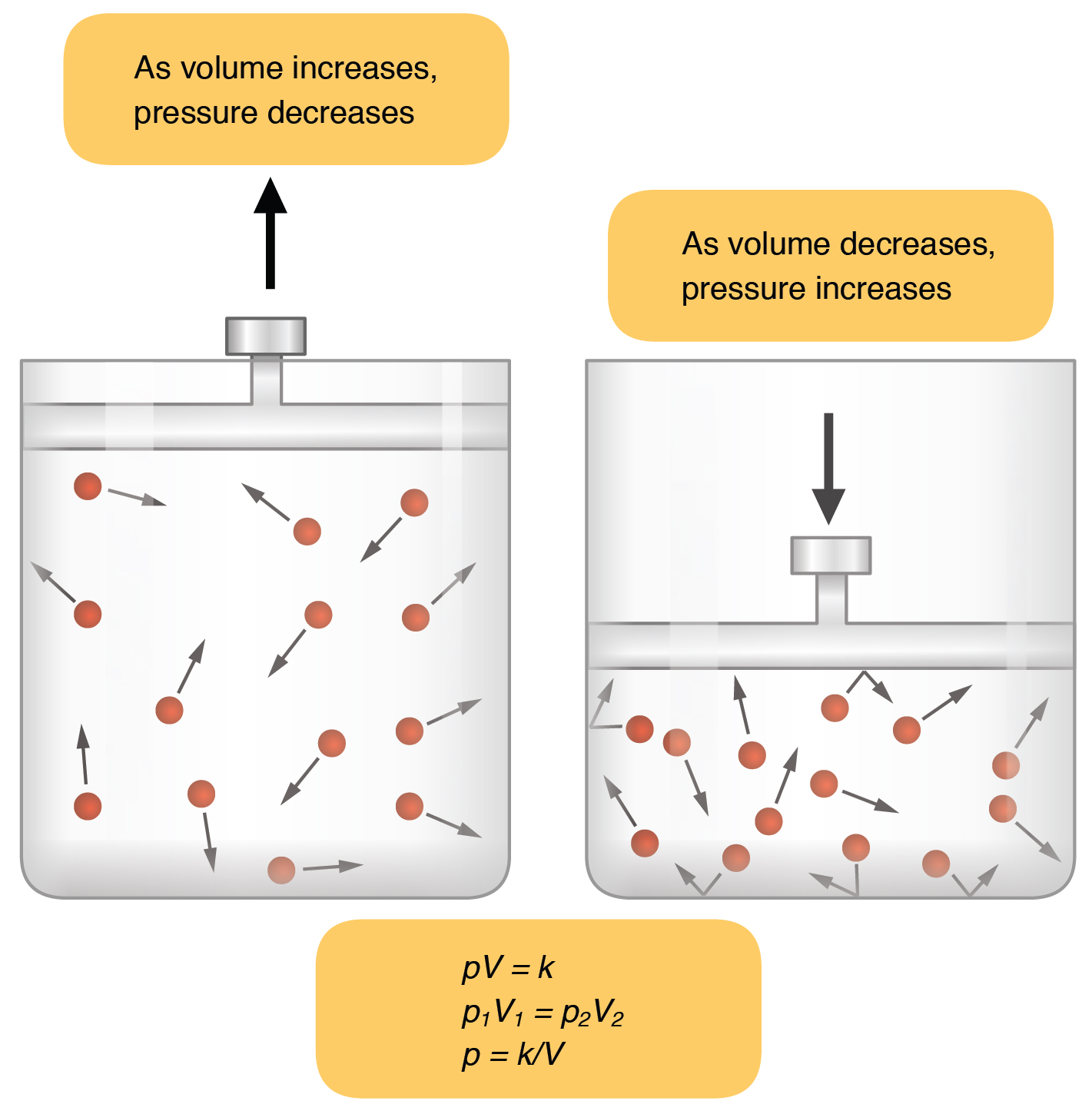
Inspiration (or inhalation) and expiration (or exhalation) are dependent on the differences in pressure between the atmosphere and the lungs. In a gas, pressure is a force created by the movement of gas molecules that are confined. For example, a certain number of gas molecules in a two-liter container has more room than the same number of gas molecules in a one-liter container (Figure 62.1). In this case, the force exerted by the movement of the gas molecules against the walls of the two-liter container is lower than the force exerted by the gas molecules in the one-liter container. Therefore, the pressure is lower in the two-liter container and higher in the one-liter container. At a constant temperature, changing the volume occupied by the gas changes the pressure, as does changing the number of gas molecules. Boyle’s law describes the relationship between volume and pressure in a gas at a constant temperature. Boyle discovered that the pressure of a gas is inversely proportional to its volume: If volume increases, pressure decreases. Likewise, if volume decreases, pressure increases. Pressure and volume are inversely related (P = k/V). Therefore, the pressure in the one-liter container (one-half the volume of the two-liter container) would be twice the pressure in the two-liter container. Boyle’s law is expressed by the following formula:
In this formula, P1 represents the initial pressure and V1 represents the initial volume, whereas the final pressure and volume are represented by P2 and V2, respectively. If the two- and one-liter containers were connected by a tube and the volume of one of the containers were changed, then the gases would move from higher pressure (lower volume) to lower pressure (higher volume).
Physical Factors Affecting Ventilation
In addition to the differences in pressures, breathing is also dependent upon the contraction and relaxation of muscle fibers of both the diaphragm and thorax. The lungs themselves are passive during breathing, meaning they are not involved in creating the movement that helps inspiration and expiration. This is because of the adhesive nature of the pleural fluid, which allows the lungs to be pulled outward when the thoracic wall moves during inspiration. The recoil of the thoracic wall during expiration causes compression of the lungs. Contraction and relaxation of the diaphragm and intercostal muscles (found between the ribs) cause most of the pressure changes that result in inspiration and expiration. These muscle movements and subsequent pressure changes cause air to either rush in or be forced out of the lungs.
Other characteristics of the lungs influence the effort that must be expended to ventilate. Resistance is a force that slows motion, in this case, the flow of gases. The size of the airway is the primary factor affecting resistance. A small tubular diameter forces air through a smaller space, causing more collisions of air molecules with the walls of the airways. The following formula helps to describe the relationship between airway resistance and pressure changes:
As noted earlier, there is surface tension within the alveoli caused by water present in the lining of the alveoli. This surface tension tends to inhibit expansion of the alveoli. However, pulmonary surfactant secreted by type II alveolar cells mixes with that water and helps reduce this surface tension. Without pulmonary surfactant, the alveoli would collapse during expiration.
Thoracic wall compliance is the ability of the thoracic wall to stretch while under pressure. This can also affect the effort expended in the process of breathing. In order for inspiration to occur, the thoracic cavity must expand. The expansion of the thoracic cavity directly influences the capacity of the lungs to expand. If the tissues of the thoracic wall are not very compliant, it will be difficult to expand the thorax to increase the size of the lungs.
Adapted from Anatomy & Physiology by Lindsay M. Biga et al, shared under a Creative Commons Attribution-ShareAlike 4.0 International License, chapter 22
gas law stating that the pressure of a gas is inversely proportional to its volume when temperature is kept constant
the ability of the thoracic wall to stretch while under pressure

