72 Glomerular filtration rate (GFR)
Learning Objectives
After reading this section you should be able to-
- Define glomerular filtration rate (GFR) and explain the role of blood pressure, capsule fluid pressure, and colloid osmotic (oncotic) pressure in determining GFR.
- Describe factors that can change blood pressure, capsule fluid pressure, and colloid osmotic (oncotic) pressure and thereby change glomerular filtration rate (GFR).
- Explain the role of the juxtaglomerular apparatus (JGA) in tubuloglomerular feedback.
- Trace the changes in filtrate osmolarity as it passes through the segments of the nephron.
- Explain the role of the nephron loop (of Henle), its permeability to water, and the high osmolarity of the interstitial fluid in the renal medulla in the formation of dilute urine
Factors Determining GFR
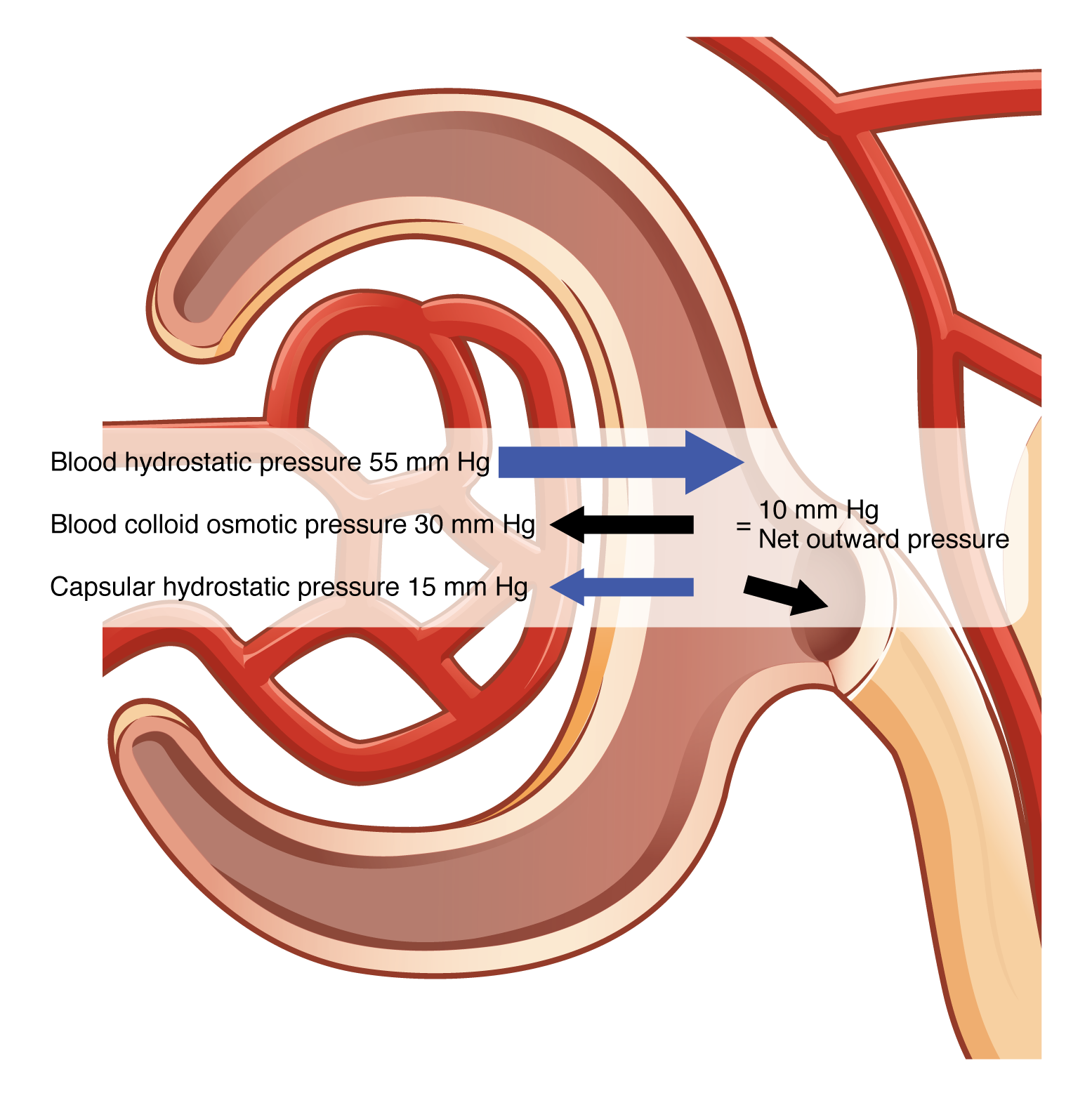
Glomerular filtration rate (GFR) is influenced by three main pressures: blood pressure (glomerular hydrostatic pressure), capsule fluid pressure (capsular hydrostatic pressure), and colloid osmotic (oncotic) pressure.
Blood pressure (glomerular hydrostatic pressure) is the force exerted by the blood against the walls of the glomerular capillaries. It promotes the filtration of blood by pushing water and solutes through the filtration membrane into the Bowman’s capsule.
Capsular fluid pressure (capsular hydrostatic pressure) is the pressure exerted by the filtrate already in the Bowman’s capsule. This pressure opposes the glomerular hydrostatic pressure, reducing the net filtration rate.
Colloid osmotic (oncotic) pressure is the pressure exerted by proteins (mainly albumin) in the blood plasma. It opposes the glomerular hydrostatic pressure by drawing water back into the glomerular capillaries from the filtrate.
NFP = Glomerular blood hydrostatic pressure (GBHP) – [capsular hydrostatic pressure (CHP) + blood colloid osmotic pressure (BCOP)] = 10 mm Hg
That is: NFP = GBHP – [CHP + BCOP] = 10 mm Hg
Or: NFP = 55 – [15 + 30] = 10 mm Hg (Figure 72.1).
Worked example: GBHP = 55 mm Hg; CHP = 15 mm Hg; BCOP = 30 mm Hg →
NFP = 55 – (15 + 30) = +10 mm Hg, indicating net filtration into Bowman’s capsule.
Factors Influencing Blood Pressure, Capsule Fluid Pressure, and Colloid Osmotic Pressure
Factors that can change these pressures include systemic blood pressure, protein levels in the blood, and obstructions in the urinary system. High blood pressure increases glomerular hydrostatic pressure, raising GFR, while low blood pressure has the opposite effect. Decreased protein levels reduce colloid osmotic pressure, increasing GFR. Obstructions in the urinary system increase capsular hydrostatic pressure, reducing GFR.
Role of the Juxtaglomerular Apparatus (JGA) in Tubuloglomerular Feedback
The juxtaglomerular apparatus (JGA) is a specialized structure formed by the distal convoluted tubule and the afferent arteriole. It helps regulate blood pressure and GFR through tubuloglomerular feedback. Macula densa cells in the distal convoluted tubule monitor sodium chloride concentration in the filtrate. When sodium chloride levels are high, these cells signal juxtaglomerular cells to release renin, which leads to the production of angiotensin II, causing vasoconstriction of the afferent arteriole and reducing GFR. This feedback mechanism maintains GFR within optimal ranges.
Changes in Filtrate Osmolarity in the Nephron
As the filtrate passes through the nephron, its osmolarity changes significantly. In the proximal convoluted tubule, water and solutes are reabsorbed, keeping the osmolarity similar to blood plasma. In the descending limb of the nephron loop (Loop of Henle), water is reabsorbed, increasing filtrate osmolarity. The ascending limb is impermeable to water but actively reabsorbs sodium and chloride, decreasing filtrate osmolarity. In the distal convoluted tubule and collecting duct, further reabsorption and secretion fine-tune the osmolarity of the filtrate.
measure of fluid that is filtered from the glomerulus into Bowman's capsule per minute
force exerted by the blood against the walls of the glomerular capillaries
pressure exerted by the filtrate already in the Bowman’s capsule
pressure exerted by proteins (mainly albumin) in the blood plasma
the sum of hydrostatic and osmotic pressures

