11 Cellular respiration
Learning Objectives
After reading this section you should be able to-
- Define the term cellular respiration
- With respect to glycolysis, the Krebs cycle, and the electron transport chain, compare and contrast
- Energy input – Is energy required to make more energy?
- Efficiency of energy production – How many ATP are produced
- Oxygen use – Is oxygen required?
- By-products (CO2, FADH2, NADH)
- Cellular location
- Explain why oxygen is necessary for energy production
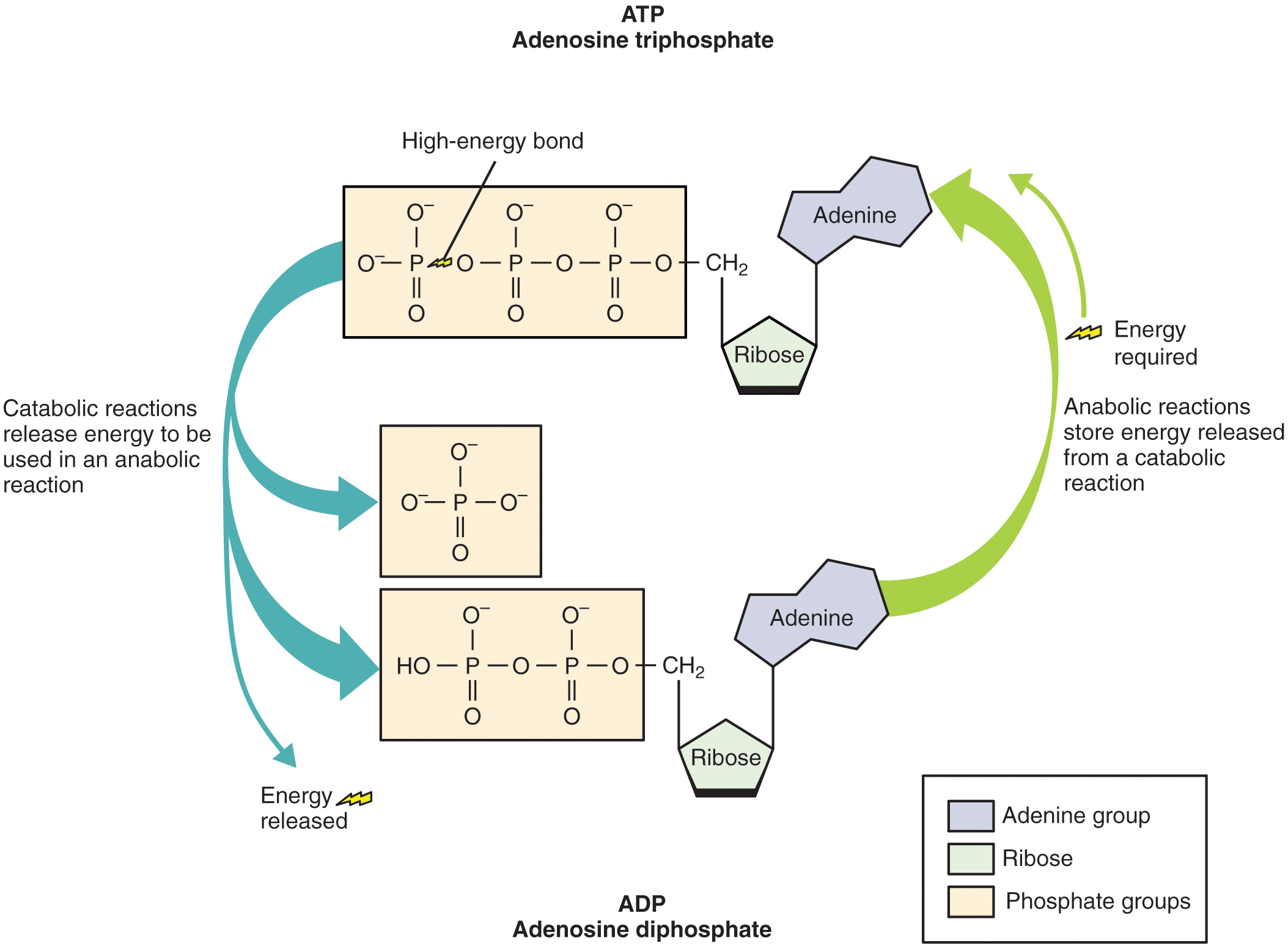
Adenosine triphosphate (ATP)
Adenosine triphosphate (ATP), the energy currency of cells, can be used immediately to power molecular machines that support cells, tissues, and organ function. This includes building new tissue and repairing damaged tissue. ATP can also be stored to fulfill future energy demands.
Structurally, ATP molecules consist of an adenine, a ribose, and three phosphate groups (Figure 11.1). The chemical bond between the second and third phosphate groups, termed a high-energy bond, represents the greatest source of energy in a cell. It is the first bond that catabolic enzymes break when cells require energy to do work. The products of this reaction are a molecule of adenosine diphosphate (ADP) and a lone phosphate group (Pi). ATP, ADP, and Pi are constantly being cycled through reactions that build ATP and store energy, and reactions that break down ATP and release energy. Maintaining low [ATP] minimizes waste and prevents futile cycling
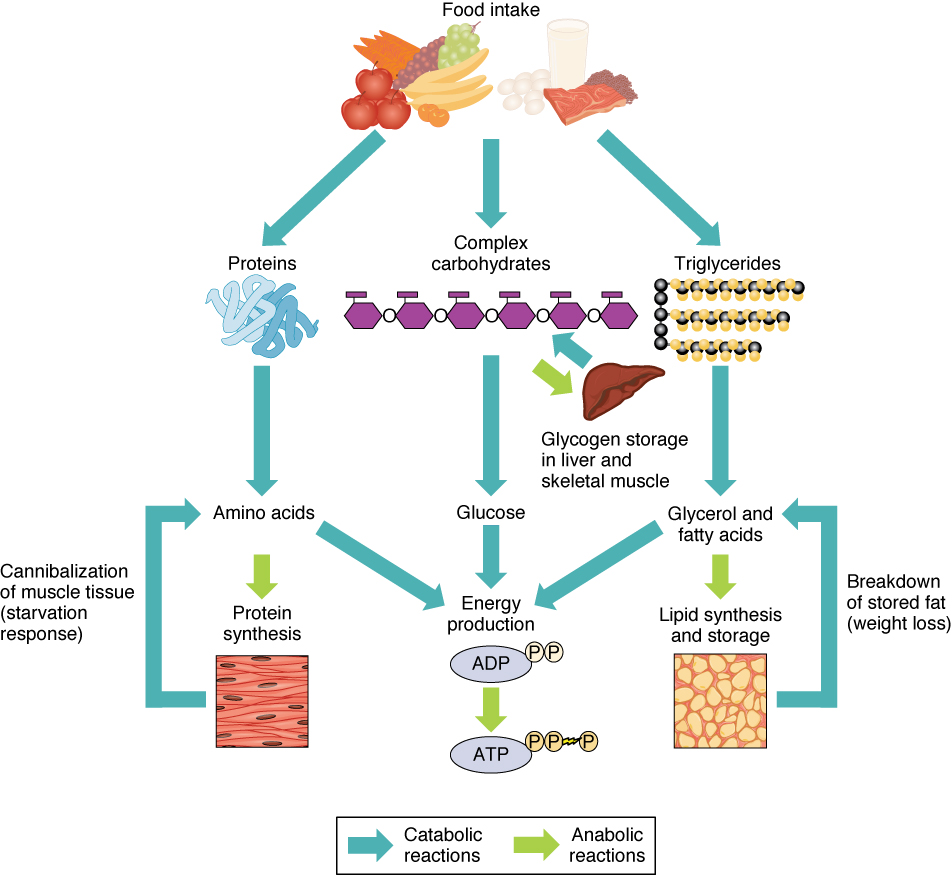
The energy from ATP drives all bodily functions, such as contracting muscles, maintaining the electrical potential of nerve cells, and absorbing food in the gastrointestinal tract. The metabolic reactions that produce ATP come from various sources (Figure 11.2).
Next, we’ll see how glycolysis and the mitochondrion regenerate ATP from ADP—so keep this reaction in mind as we move through each stage.
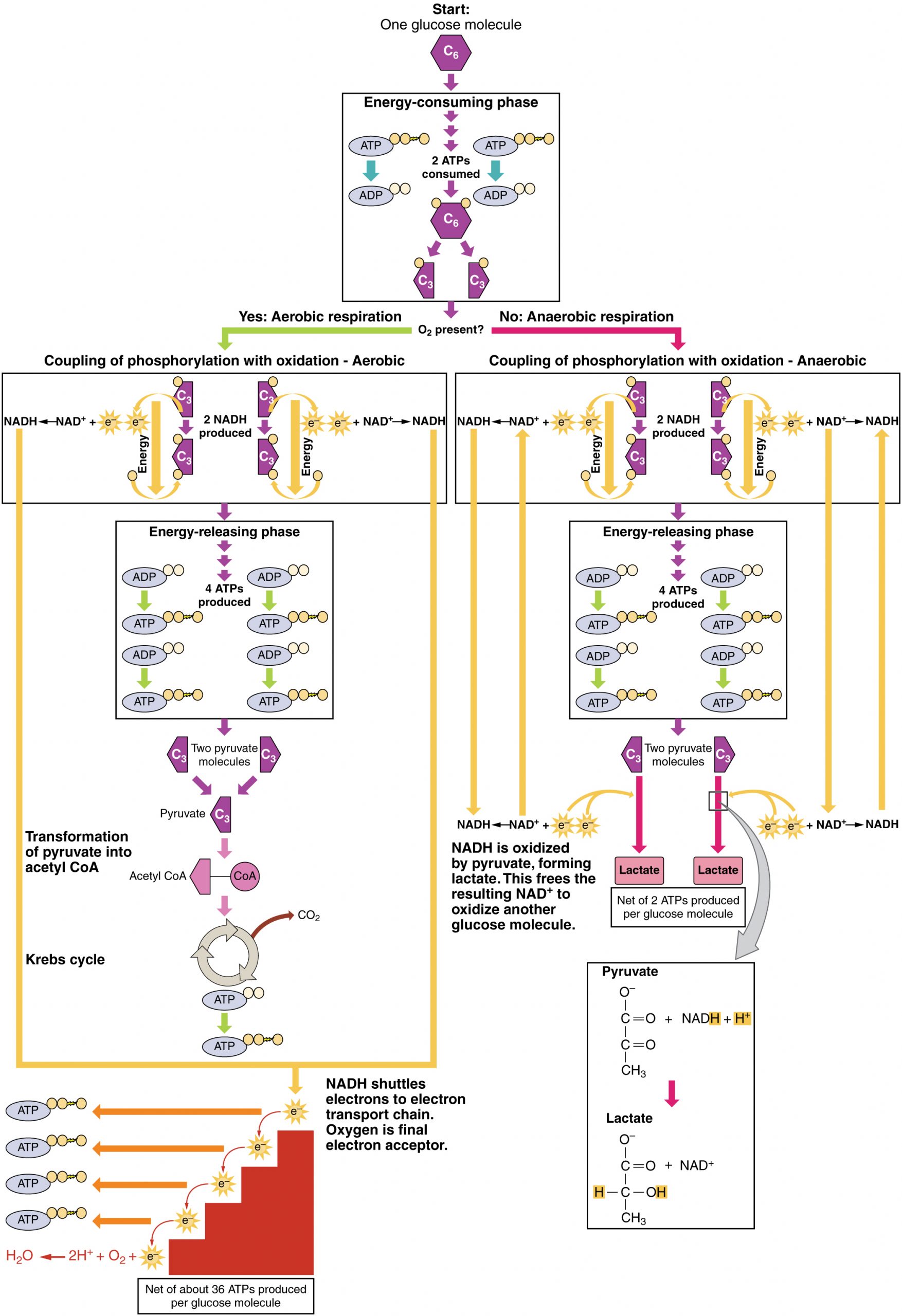
Cellular respiration
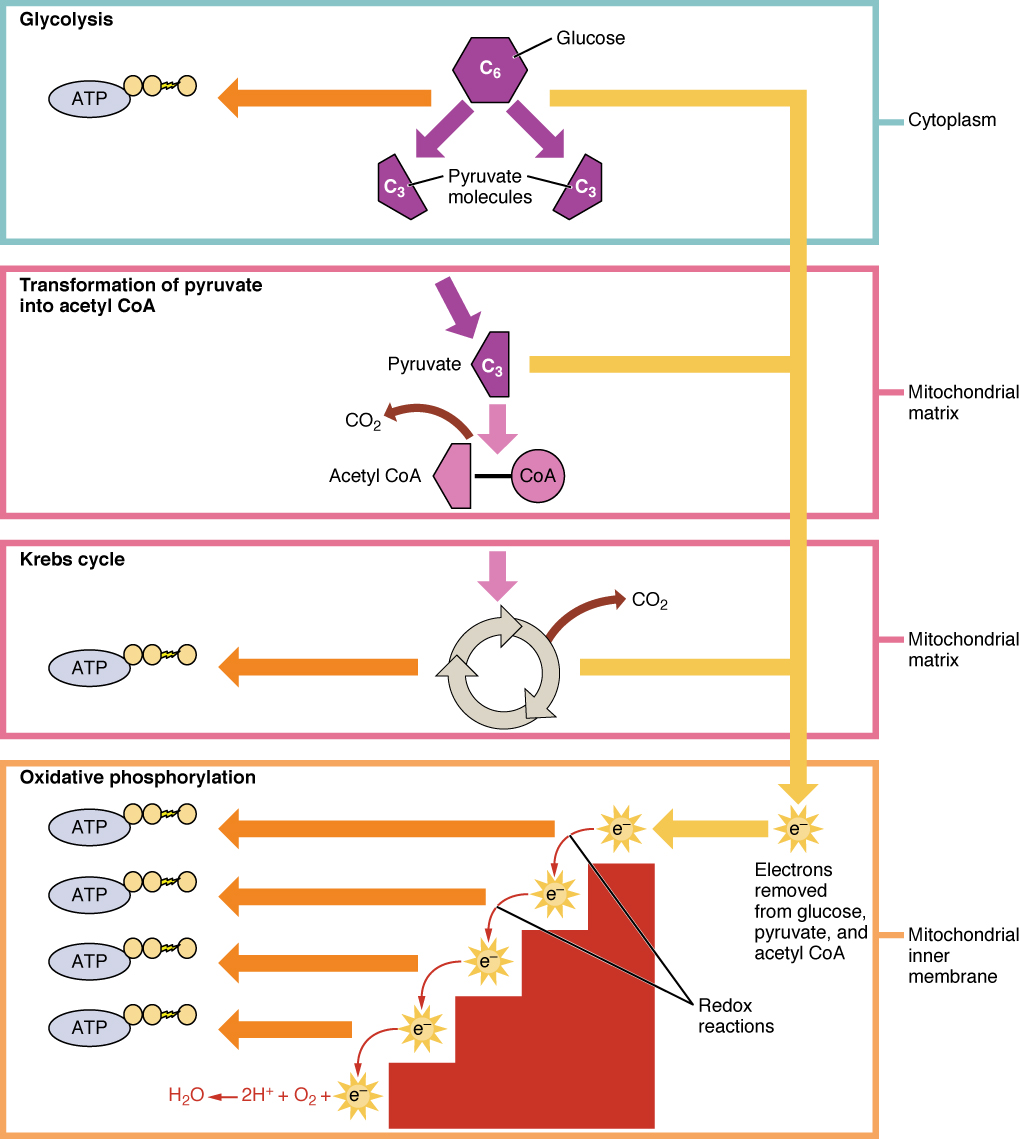
Cellular respiration is the process by which cells convert glucose and oxygen into ATP, water, and carbon dioxide, providing energy for cellular functions. The goal of cellular respiration is to produce ATP for use by the body to power physiological processes. To start the process, a glucose molecule will be modified to form two pyruvate molecules in the metabolic pathway called glycolysis. When oxygen is available, the pyruvate molecules will then be converted to acetyl CoA, which enters the mitochondria and joins the citric acid cycle. Both glycolysis and the citric acid cycle produce a small amount of ATP (2 ATP per pathway), but the majority of the ATP produced by aerobic metabolism (i.e., metabolism that requires oxygen) is achieved when the products of glycolysis and the citric acid, NADH and FADH2, carry their electrons to the electron transport chain. The electron transport chain transfers electrons through electron carriers, and onto oxygen, in a process called oxidative phosphorylation. This final process of cellular respiration harnesses the energy delivered by NADH and FADH2 to drive ATP synthase to produce 34 ATP per glucose. To briefly summarize, glycolysis yields 2 ATP per glucose molecule, the Krebs cycle yields 2 ATP, while the electron transport chain is highly efficient, producing about 34 ATP per glucose molecule. This first section will focus first on glycolysis, a process where the monosaccharide glucose is oxidized, releasing the energy stored in its bonds to produce ATP.
Glycolysis
Glucose is the body’s most readily available source of energy. After digestive processes break polysaccharides down into monosaccharides, including glucose, the monosaccharides are transported across the wall of the small intestine and into the circulatory system, which transports them to the liver. In the liver, hepatocytes either pass the glucose on through the circulatory system or store excess glucose as glycogen. Cells in the body take up the circulating glucose in response to insulin and, through a series of reactions called glycolysis, transfer some of the energy in glucose to ADP to form ATP (Figure 11.4). The last step in glycolysis produces the product pyruvate.
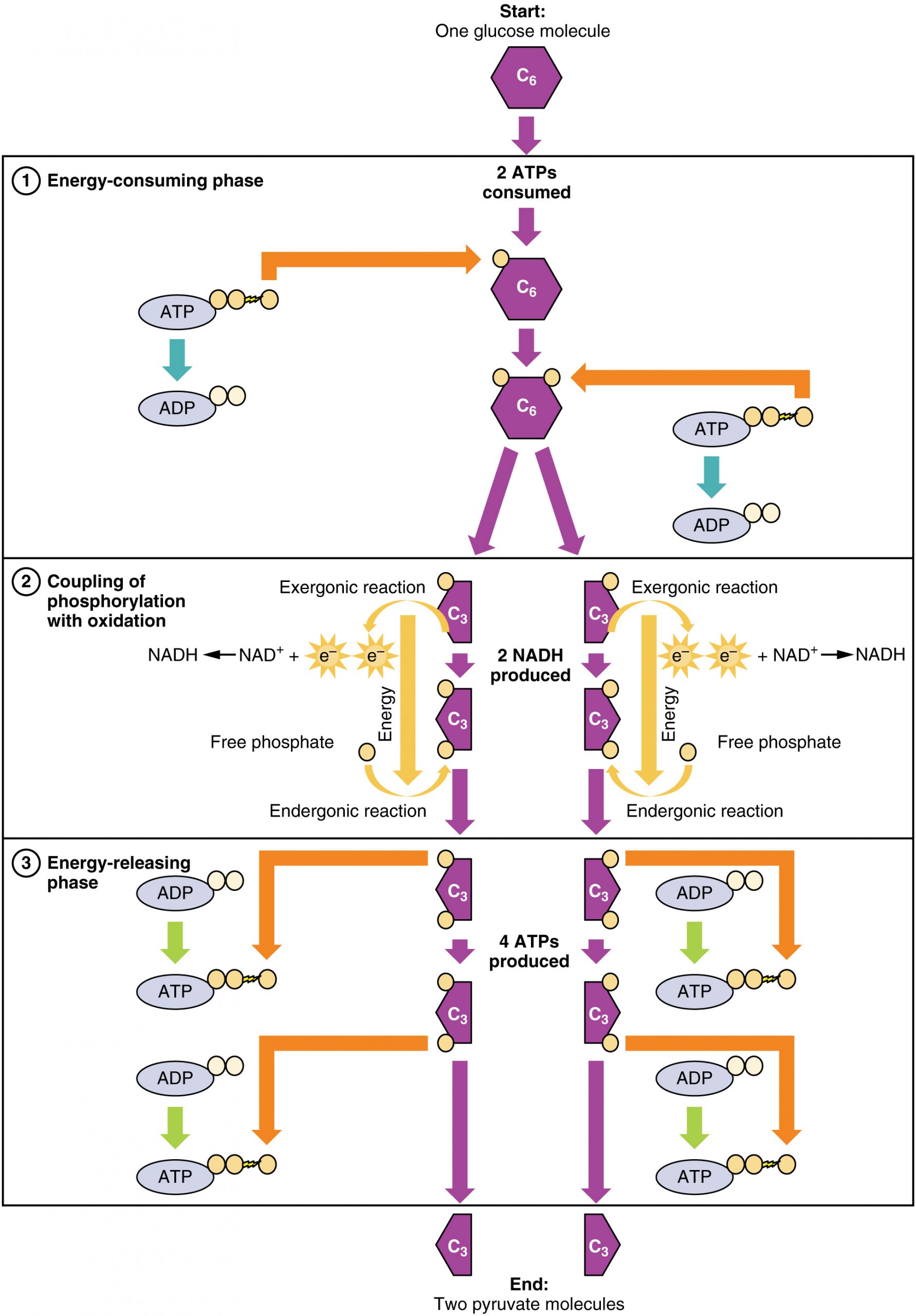
Glycolysis can be divided into two phases: energy consuming and energy yielding. The first phase is the energy-consuming phase, so it requires two ATP molecules to start the reaction for each molecule of glucose. However, the end of the reaction produces four ATPs, resulting in a net gain of two ATP energy molecules.
Glycolysis can be expressed as the following equation:
This equation states that during glycolysis, a single glucose molecules combines with ATP (the energy source), NAD+ (a coenzyme that serves as an electron acceptor), and inorganic phosphate, breaks down into two pyruvate molecules, generating four ATP molecules—for a net yield of two ATP—and two energy-containing NADH coenzymes. The NADH that is produced in this process will be used later to produce ATP in the mitochondria. Importantly, by the end of this process, one glucose molecule generates two pyruvate molecules, two high-energy ATP molecules, and two electron-carrying NADH molecules.
The following discussions of glycolysis include the enzymes responsible for the reactions. When glucose enters a cell, the enzyme hexokinase rapidly adds a phosphate to convert it into glucose-6-phosphate. A kinase is a type of enzyme that adds a phosphate molecule to a substrate. This conversion step requires one ATP and essentially traps the glucose in the cell, preventing it from passing back through the plasma membrane, thus allowing glycolysis to proceed. It also functions to maintain a concentration gradient and ensure higher glucose levels in the blood compared to the tissues. By establishing this concentration gradient, the glucose in the blood will be able to flow from an area of high concentration (the blood) into an area of low concentration (the tissues) to be either used or stored.
In the next step of the first phase of glycolysis, the enzyme glucose-6-phosphate isomerase converts glucose-6-phosphate into fructose-6-phosphate. Like glucose, fructose is also a six carbon-containing sugar. The enzyme phosphofructokinase-1 then adds one more phosphate to convert fructose-6-phosphate into fructose-1-6-bisphosphate, another six-carbon sugar, using another ATP molecule. Aldolase then breaks down this fructose-1-6-bisphosphate into two three-carbon molecules, glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. The triosephosphate isomerase enzyme then converts dihydroxyacetone phosphate into a second glyceraldehyde-3-phosphate molecule. By the end of this chemical-priming or energy-consuming phase, one glucose molecule is broken down into two glyceraldehyde-3-phosphate molecules.
The second phase of glycolysis, the energy-yielding phase, creates the energy that is the product of glycolysis. Glyceraldehyde-3-phosphate dehydrogenase converts each three-carbon glyceraldehyde-3-phosphate produced during the energy-consuming phase into 1,3-bisphosphoglycerate. This reaction releases an electron that is then picked up by NAD+ to create an NADH molecule. NADH is a high-energy molecule, like ATP, but unlike ATP, it is not used as energy currency by the cell. Because there are two glyceraldehyde-3-phosphate molecules, two NADH molecules are synthesized during this step. Each 1,3-bisphosphoglycerate is subsequently dephosphorylated (i.e., a phosphate is removed) by phosphoglycerate kinase into 3-phosphoglycerate. Each phosphate released in this reaction can convert one molecule of ADP into one high-energy ATP molecule, resulting in a gain of two ATP molecules.
The enzyme phosphoglycerate mutase then converts the 3-phosphoglycerate molecules into 2-phosphoglycerate. The enolase enzyme then acts upon the 2-phosphoglycerate molecules to convert them into phosphoenolpyruvate molecules. The last step of glycolysis involves the dephosphorylation of the two phosphoenolpyruvate molecules by pyruvate kinase to create two pyruvate molecules and two ATP molecules.
In summary, glycolysis utilizes one glucose molecule which breaks down into two pyruvate molecules, and creates two net ATP molecules and two NADH molecules. Therefore, glycolysis generates energy for the cell and creates pyruvate molecules that can be processed further through the aerobic Krebs cycle (also called the citric acid cycle or tricarboxylic acid cycle); converted into lactate or alcohol (in yeast) by fermentation; or used later for the synthesis of glucose through gluconeogenesis. Briefly, this occurs when lactate enters the blood stream and is shuttled to the liver. Lactate undergoes the Cori cycle to produce glucose, which can re-enter the bloodstream and be transported to muscle or other tissues.
Anaerobic Respiration
When oxygen is limited or absent, pyruvate enters an anaerobic pathway. In these reactions, pyruvate can be converted into lactate. In addition to generating an additional ATP, this pathway serves to keep the pyruvate concentration low so glycolysis continues, and it oxidizes NADH into the NAD+ needed by glycolysis. In this reaction, lactate replaces oxygen as the final electron acceptor. Anaerobic respiration occurs in most cells of the body when oxygen is limited or mitochondria are absent or nonfunctional. For example, because erythrocytes (red blood cells) lack mitochondria, they must produce their ATP from anaerobic respiration. This is an effective pathway of ATP production for short periods of time, ranging from seconds to a few minutes. The lactate produced diffuses into the plasma and is carried to the liver, where it is converted back into pyruvate or glucose via the Cori cycle. Similarly, when a person exercises, muscles use ATP faster than oxygen can be delivered to them. Muscles ultimately depend on glycolysis and lactate production for rapid ATP production.
Aerobic Respiration
In the presence of oxygen, pyruvate can enter the Krebs cycle where additional energy is extracted as electrons are transferred from the pyruvate to the receptors NAD+, GDP, and FAD, with carbon dioxide being a “waste product” (Figure 11.5). The NADH and FADH2 pass electrons on to the electron transport chain, which uses the transferred energy to produce ATP. As the terminal step in the electron transport chain, oxygen is the terminal electron acceptor and creates water inside the mitochondria.
Krebs Cycle/Citric Acid Cycle/Tricarboxylic Acid Cycle
The pyruvate molecules generated during glycolysis are transported across the mitochondrial membrane into the inner mitochondrial matrix, where they are metabolized by enzymes in a pathway called the Krebs cycle (Figure 11.6). The Krebs cycle is also commonly called the citric acid cycle or the tricarboxylic acid (TCA) cycle. During the Krebs cycle, high-energy molecules, including ATP, NADH, and FADH2, are created. NADH and FADH2 then pass electrons through the electron transport chain in the mitochondria to generate more ATP molecules.
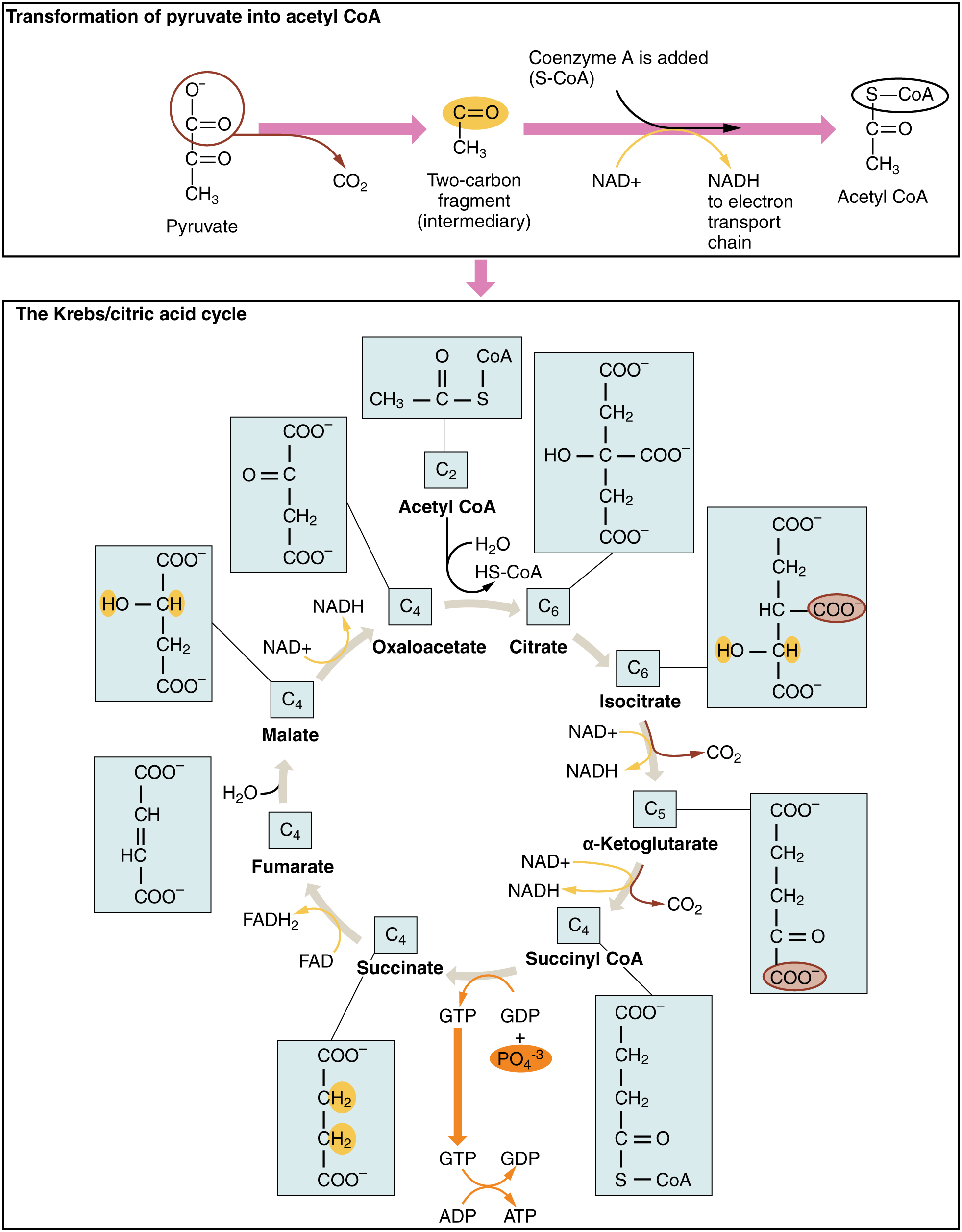
The three-carbon pyruvate molecule generated during glycolysis moves from the cytoplasm into the mitochondrial matrix, where it is converted by the enzyme pyruvate dehydrogenase into a two-carbon acetyl coenzyme A (acetyl CoA) molecule. This reaction is an oxidative decarboxylation reaction. It converts the three-carbon pyruvate into a two-carbon acetyl CoA molecule, releasing carbon dioxide and transferring two electrons that combine with NAD+ to form NADH. Acetyl CoA enters the Krebs cycle by combining with a four-carbon molecule, oxaloacetate, to form the six-carbon molecule citrate, or citric acid, at the same time releasing the coenzyme A molecule.
The six-carbon citrate molecule is systematically converted to a five-carbon molecule and then a four-carbon molecule, ending with oxaloacetate, the beginning of the cycle. Along the way, each citrate molecule will produce one ATP, one FADH2, and three NADH. The FADH2 and NADH will enter the oxidative phosphorylation system located in the inner mitochondrial membrane. In addition, the Krebs cycle supplies the starting materials to process and break down proteins and fats.
To start the Krebs cycle, citrate synthase combines acetyl CoA and oxaloacetate to form a six-carbon citrate molecule; CoA is subsequently released and can combine with another pyruvate molecule to begin the cycle again. The aconitase enzyme converts citrate into isocitrate. In two successive steps of oxidative decarboxylation, two molecules of CO2 and two NADH molecules are produced when isocitrate dehydrogenase converts isocitrate into the five-carbon α-ketoglutarate, which is then catalyzed and converted into the four-carbon succinyl CoA by α-ketoglutarate dehydrogenase. The enzyme succinyl CoA dehydrogenase then converts succinyl CoA into succinate and forms the high-energy molecule GTP, which transfers its energy to ADP to produce ATP. Succinate dehydrogenase then converts succinate into fumarate, forming a molecule of FADH2. Fumarase then converts fumarate into malate, which malate dehydrogenase then converts back into oxaloacetate while reducing NAD+ to NADH. Oxaloacetate is then ready to combine with the next acetyl CoA to start the Krebs cycle again. For each turn of the cycle, three NADH, one ATP (through GTP), and one FADH2 are created. Each carbon of pyruvate is converted into CO2, which is released as a byproduct of oxidative (aerobic) respiration.
Oxidative Phosphorylation and the Electron Transport Chain
The electron transport chain (ETC) uses the NADH and FADH2 produced by the Krebs cycle to generate ATP. Electrons from NADH and FADH2 are transferred through protein complexes embedded in the inner mitochondrial membrane by a series of enzymatic reactions. The electron transport chain consists of a series of four enzyme complexes (Complex I – Complex IV) and two coenzymes (ubiquinone and Cytochrome c), which act as electron carriers and proton pumps used to transfer H+ ions into the space between the inner and outer mitochondrial membranes (Figure 11.7). The ETC couples the transfer of electrons between a donor (like NADH) and an electron acceptor (like O2) with the transfer of protons (H+ ions) across the inner mitochondrial membrane, enabling the process of oxidative phosphorylation. In the presence of oxygen, energy is passed, stepwise, through the electron carriers to collect gradually the energy needed to attach a phosphate to ADP and produce ATP. Molecular oxygen, O2 acts as the terminal electron acceptor for the ETC. This means that once the electrons have passed through the entire ETC, they must be passed to another, separate molecule. These electrons, O2, and H+ ions from the matrix combine to form new water molecules. This is the basis for your need to breathe in oxygen. Oxygen is essential in cellular respiration because it acts as the final electron acceptor in the electron transport chain, allowing the efficient production of ATP. Without oxygen, the electron transport chain halts, and cells must rely on less efficient anaerobic processes.
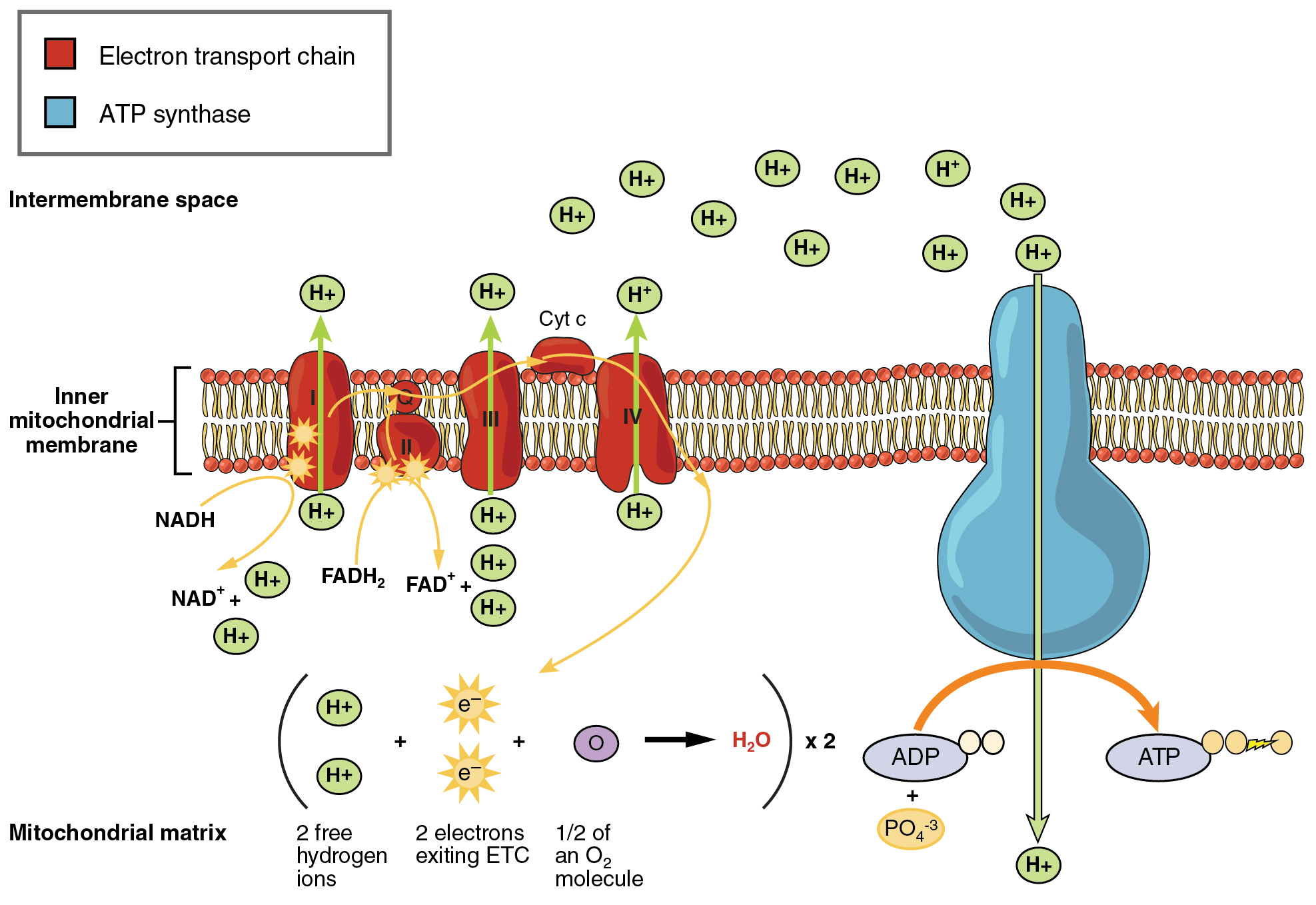
The electrons released from NADH and FADH2 are passed along the chain by each of the carriers, which are reduced when they receive the electron and oxidized when passing it on to the next carrier. Each of these reactions releases a small amount of energy, which is used to pump H+ ions across the inner membrane. The accumulation of these protons in the space between the membranes creates a proton gradient with respect to the mitochondrial matrix.
Also embedded in the inner mitochondrial membrane is an amazing protein pore complex called ATP synthase. Effectively, it is a turbine that is powered by the flow of H+ ions across the inner membrane down a gradient and into the mitochondrial matrix. As the H+ ions traverse the complex, the shaft of the complex rotates. This rotation causes ADP and Pi to be combined which creates ATP. In accounting for the total number of ATP produced per glucose molecule through aerobic respiration, it is important to remember the following points:
- A net of two ATP are produced through glycolysis (four produced and two consumed during the energy-consuming stage). However, these two ATP are used for transporting the NADH produced during glycolysis from the cytoplasm into the mitochondria. Therefore, the net production of ATP during glycolysis is zero.
- In all phases after glycolysis, the number of ATP, NADH, and FADH2 produced must be multiplied by two to reflect how each glucose molecule produces two pyruvate molecules.
- In the ETC, about three ATP are produced for every oxidized NADH. However, only about two ATP are produced for every oxidized FADH2. The electrons from FADH2 produce less ATP, because they start at a lower point in the ETC (Complex II) compared to the electrons from NADH (Complex I).
Therefore, for every glucose molecule that enters aerobic respiration, a net total of 36 ATPs are produced (Figure 11.8). For a concise, side-by-side comparison of all four stages—including location, net ATP, O₂ requirement, and by-products—see Table 11.1 below.
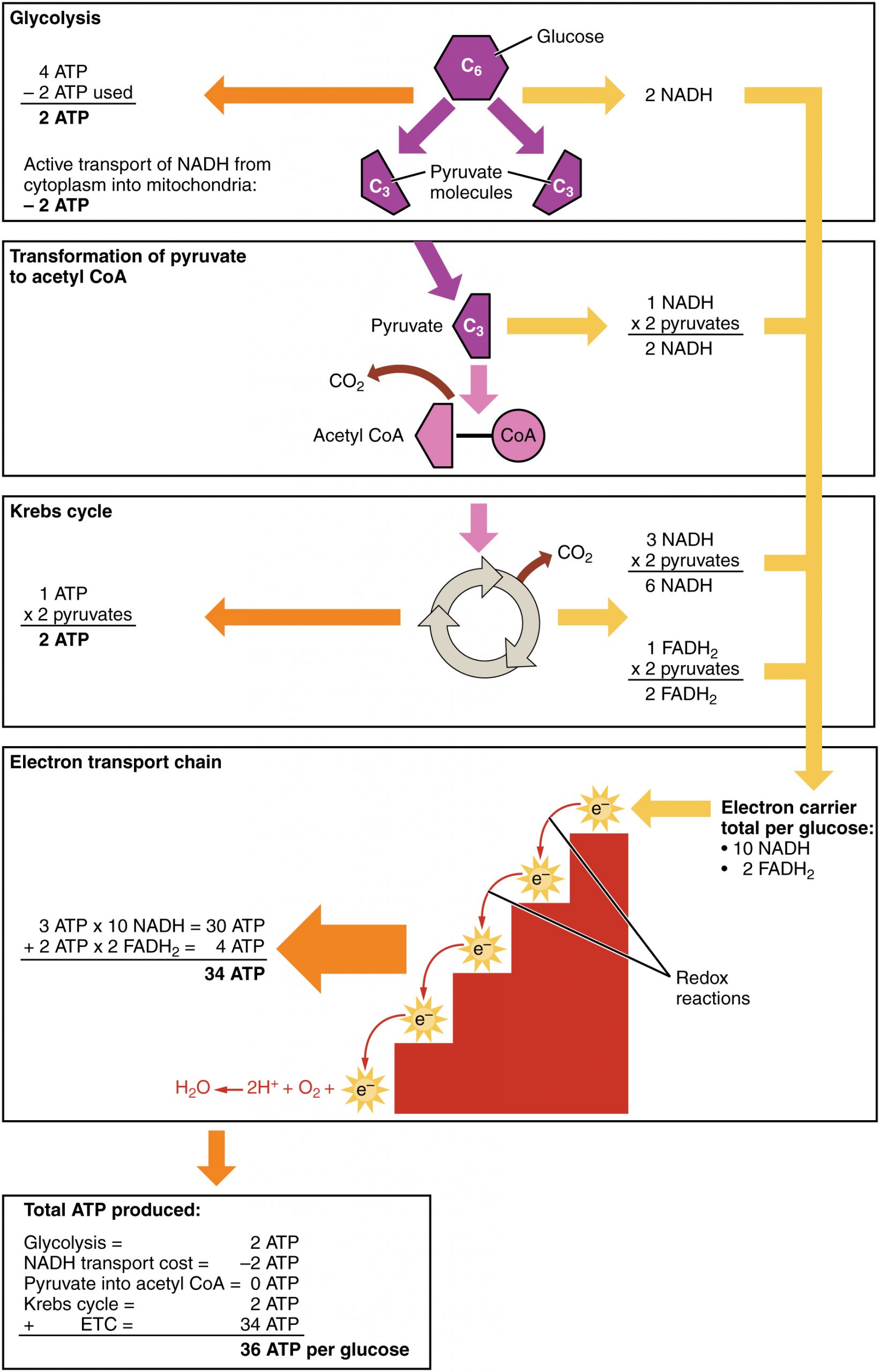
Table 11.1 – Comparison of the Stages of Cellular Respiration
| Stage | Location | ATP Yield (net) | O2 required | CO2 produced | NADH (net) | FADH2 (Net) |
| Glycolysis | Cytoplasm | +2 | No | No | 2 | 0 |
| Pyrvuate → Acteyl CoA | Mitochondrial matrix | 0 | No 2 | Yes | 2 | 0 |
| Krebs cycle | Mitochondrial matrix | +2 | No 3 | Yes | 6 | 2 |
| Electron transport chain | Inner mitochondrial membrane | ~28 1 | Yes | No | 0 (ETC uses NADH; net is zero) | 0 (ETC uses FADH2; net is zero) |
¹ modern estimates use P/O (phosphate to oxygen ratio) (≈2.5 ATP/NADH, 1.5 ATP/FADH₂) rather than the classic “3 + 2”
² oxygen isn’t used directly but is required downstream to regenerate NAD⁺
³ same as above—O₂ prevents back-up of electrons into fermentation
Adapted from Anatomy & Physiology by Lindsay M. Biga et al, shared under a Creative Commons Attribution-ShareAlike 4.0 International License, chapter 24.
nucleotide responsible for providing energy in living cells, composed of an adenine base, ribose, and three phosphate groups
the anaerobic metabolic pathway occurring within the cytoplasm of a cell that splits a molecule of glucose into two pyruvate molecules
a series of chemical reactions that occur in the inner mitochondrial membrane in which electrons are moved across the membrane and ATP is produced
also known as the citric acid cycle and/or tricarboxylic acid cycle; the sequence of chemical reactions in the mitochondrial matrix that oxidize acetyl coenzyme A and produce NADH, FADH2, and ATP
the intermediate of metabolic pathways, formed from glycolysis
the stage of glycolysis in which ATP is used to break glucose down into two three-carbon molecules
an intermediate in glucose metabolism, formed by from the phosphorylation of glucose by hexokinase
the second phase of glycolysis, during which ATP is produced
metabolic process occurring in the liver and kidneys during which non-carbohydrate sources are utilized to form glucose
metabolic pathway in the liver during which lactate is converted into glucose
oxygen in the electron transport chain; the last compound to receive an electron from another compound
a molecule that participates in the Krebs Cycle by providing acetyl groups to be oxidized
the process of ATP formation through the transfer of electrons by electron carriers
a protein pore complex powered by the movement of hydrogen ions down a gradient, located in the inner mitochondrial membrane, that encourages ADP and Pi to create ATP

