35 Cross bridge-cycling
Learning Objectives
After reading this section, you should be able to-
- Define the sliding filament theory of skeletal muscle contraction.
- Describe the sequence of events involved in the contraction of a skeletal muscle fiber, including events at the neuromuscular junction, excitation-contraction coupling, and cross-bridge cycling.
- Describe the sequence of events involved in skeletal muscle relaxation.
As you have learned, during contraction, the myosin heads of the thick filament bind to actin and pull the thin filament, which shortens the sarcomere and produces force. However, the length of the myosin hinge region allows each myosin head to only pull a very short distance before it must reset to pull again. For thin filaments to continue to slide past thick filaments during muscle contraction, myosin heads must pull the actin at the binding sites, detach, re-cock, attach to more binding sites, pull, detach, re-cock, etc. This repeated movement is known cross-bridge cycling and is dependent on ATP (Figure 35.1).
Recall that each myosin head has a region that binds to actin and a region that binds to ATP. Myosin cannot release from actin until ATP binds, and the hydrolysis of ATP into adenosine diphosphate (ADP) and inorganic phosphate (Pi) then releases energy needed for the myosin head to reposition or re-cock. Cross-bridge formation occurs when the myosin head attaches to actin while ADP and Pi are still bound to myosin. Pi is then released, causing the myosin head to move toward the M-line, pulling the actin along with it. This movement is called the power stroke. ADP is then released, resulting in a stronger attachment of myosin to actin, referred to as the rigor state. In the absence of ATP, the myosin head will not detach from actin. ATP binding causes the myosin head to detach from actin. After detachment, ATP is hydrolyzed into ADP and Pi by the myosin ATPase activity. The energy released during ATP hydrolysis re-cocks the myosin head, preparing it for the next cycle of attachment and pulling. Each thick filament has multiple myosin heads that cycle asynchronously to maintain constant tension in the activated myofiber. During muscle contraction, many cross-bridges form and break continuously. The energy demand for this process is high, explaining the need for ATP. In the absence of ATP, as seen in rigor mortis, myosin heads remain bound to actin, causing muscle rigidity.
Contraction of a Skeletal Muscle
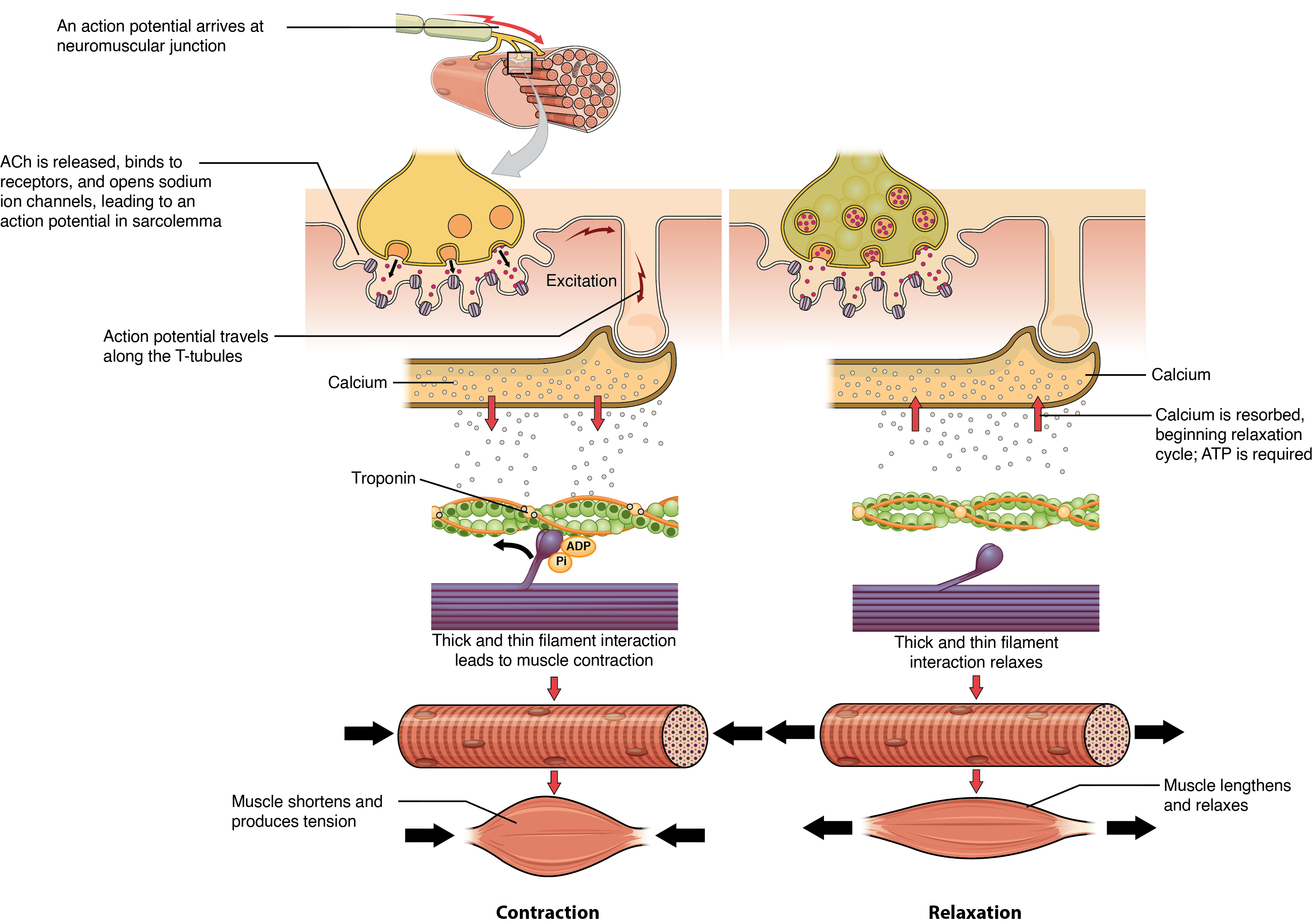
The sequence of events that result in muscle fiber contraction begins with the neurotransmitter ACh from the motor neuron. ACh binds to receptors on the muscle fiber’s sarcolemma, causing depolarization and triggering an action potential that spreads along the sarcolemma and T-tubules. This leads to the release of Ca²⁺ ions from the sarcoplasmic reticulum (SR). Ca²⁺ binds to troponin, unshielding the actin-binding sites, allowing myosin heads to form cross-bridges and pull the actin filaments (Figure 35.2). As long as Ca²⁺ and ATP are present, cross-bridge cycling continues, and the muscle fiber shortens.
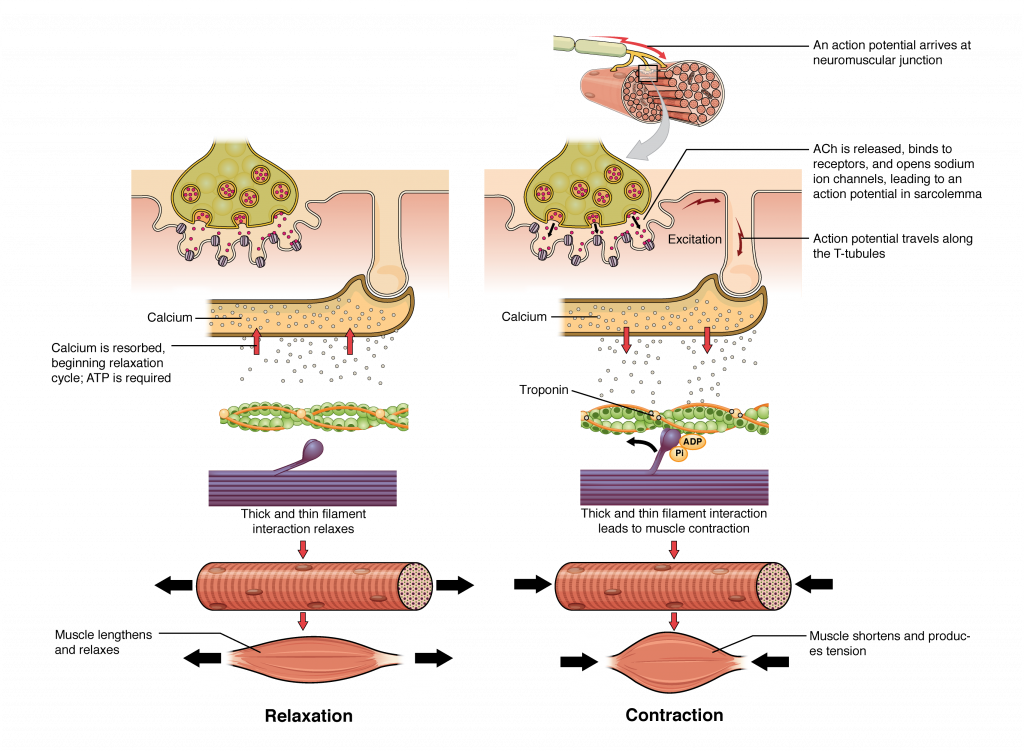
Relaxation of a Skeletal Muscle
Relaxation begins when the motor neuron stops releasing ACh into the synapse at the NMJ. The muscle fiber repolarizes, closing the SR calcium channels. ATP-driven pumps then move Ca²⁺ back into the SR, reducing the Ca²⁺ concentration in the sarcoplasm. This causes tropomyosin to re-cover the actin-binding sites, preventing cross-bridge formation. Without cross-bridge cycling, the muscle fiber loses tension and relaxes.
Adapted from Anatomy & Physiology by Lindsay M. Biga et al, shared under a Creative Commons Attribution-ShareAlike 4.0 International License, chapter 10.
the repetitive processes of myosin heads binding to actin, pulling to form cross-bridges, detaching, and re-cocking

