66 Dalton’s law and Henry’s Law
Learning Objectives
After reading this section, you should be able to-
- Explain the relationship between the total pressure of gases in a mixture and the partial pressure of an individual gas (i.e., Dalton’s Law).
- Explain the relationship between the partial pressure of a gas in air, the solubility of that gas in water, and the amount of the gas that can dissolve in water.
- Compare and contrast the solubility of oxygen and carbon dioxide in plasma.
In order to understand the mechanisms of gas exchange in the lung, it is important to understand the underlying principles of gases and their behaviors. In addition to Boyle’s law, several other gas laws help to describe the behavior of gases.
Gas Laws and Air Composition
Gas molecules exert force on the surfaces with which they are in contact; this force is called pressure. In natural systems, gases are normally present as a mixture of different types of molecules. For example, the atmosphere consists of oxygen, nitrogen, carbon dioxide, and other gaseous molecules, and this gaseous mixture exerts a certain pressure referred to as atmospheric pressure (Table 65.1). Partial pressure (Px) is the pressure of a single type of gas in a mixture of gases. For example, in the atmosphere, oxygen exerts a partial pressure, and nitrogen exerts another partial pressure, independent of the partial pressure of oxygen (Figure 65.1). Total pressure is the sum of all the partial pressures of a gaseous mixture. Dalton’s law describes the behavior of nonreactive gases in a gaseous mixture and states that a specific gas type in a mixture exerts its own pressure; thus, the total pressure exerted by a mixture of gases is the sum of the partial pressures of the gases in the mixture.
| Partial Pressures of Atmospheric Gases (Table 65.1) | ||
|---|---|---|
| Gas | Percent of total composition | Partial pressure
(mm Hg)
|
| Nitrogen (N2) | 78.6 | 597.4 |
| Oxygen (O2) | 20.9 | 158.8 |
| Water (H2O) | 0.04 | 3.0 |
| Carbon dioxide (CO2) | 0.004 | 0.3 |
| Others | 0.0006 | 0.5 |
| Total composition/total atmospheric pressure | 100% | 760.0 |
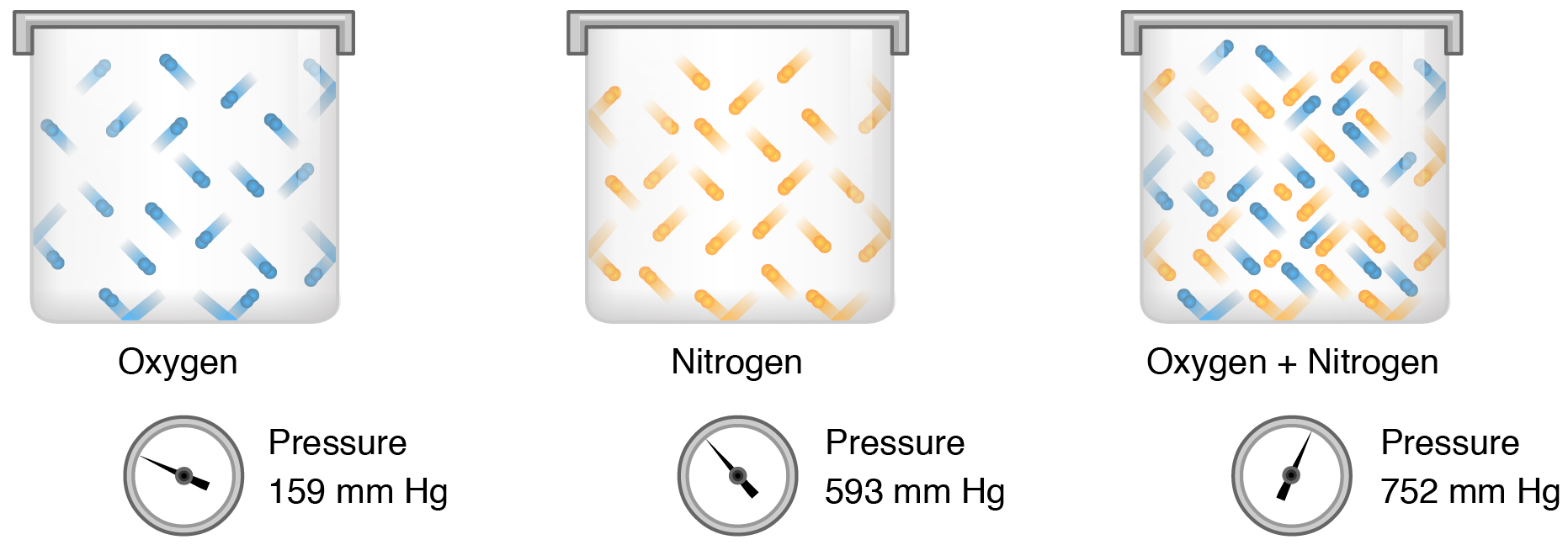
Partial pressure is extremely important in predicting the movement of gases. Recall that gases tend to equalize their pressure in two regions that are connected. A gas will move from an area where its partial pressure is higher to an area where its partial pressure is lower. In addition, the greater the partial pressure difference between the two areas, the more rapid the movement of gases is.
Solubility of Gases in Liquids
Henry’s law escribes the behavior of gases when they come into contact with a liquid, such as blood. Henry’s law states that the concentration of gas in a liquid is directly proportional to the solubility and partial pressure of that gas. The greater the partial pressure of the gas, the greater the number of gas molecules that will dissolve in the liquid. The concentration of the gas in a liquid is also dependent on the solubility of the gas in the liquid. For example, although nitrogen is present in the atmosphere, very little nitrogen dissolves into the blood, because the solubility of nitrogen in blood is very low. The exception to this occurs in scuba divers; the composition of the compressed air that divers breathe causes nitrogen to have a higher partial pressure than normal, causing it to dissolve in the blood in greater amounts than normal. Too much nitrogen in the bloodstream results in a serious condition that can be fatal if not corrected. Gas molecules establish an equilibrium between those molecules dissolved in liquid and those in air.
The composition of air in the atmosphere and in the alveoli differs. In both cases, the relative concentration of gases is nitrogen > oxygen > water vapor > carbon dioxide. The amount of water vapor present in alveolar air is greater than that in atmospheric air (Table 65.2). Recall that the respiratory system works to humidify incoming air, thereby causing the air present in the alveoli to have a greater amount of water vapor than atmospheric air. In addition, alveolar air contains a greater amount of carbon dioxide and less oxygen than atmospheric air. This is no surprise, as gas exchange removes oxygen from and adds carbon dioxide to alveolar air. Both deep and forced breathing cause the alveolar air composition to be changed more rapidly than during quiet breathing. As a result, the partial pressures of oxygen and carbon dioxide change, affecting the diffusion process that moves these materials across the membrane. This will cause oxygen to enter and carbon dioxide to leave the blood more quickly.
| Composition and Partial Pressures of Alveolar Air (Table 65.2) | ||
|---|---|---|
| Gas | Percent of total composition | Partial pressure
(mm Hg)
|
| Nitrogen (N2) | 74.9 | 569 |
| Oxygen (O2) | 13.7 | 104 |
| Water (H2O) | 6.2 | 40 |
| Carbon dioxide (CO2) | 5.2 | 47 |
| Total composition/total alveolar pressure | 100% | 760.0 |
Comparing the Solubility of Oxygen and Carbon Dioxide in Plasma
gas law stating that the pressure of a gas is inversely proportional to its volume when temperature is kept constant
the amount of force that is exerted by gases in the air surrounding any given surface, such as the body, expressed in millimeters of mercury (mmHg) or atmospheres (atm); 760 mmHg at sea level
the pressure of a single type of gas in a mixture of gases
the sum of all partial pressures of gases in a mixture
gas law stating that the total pressure exerted by a mixture of gases is the sum of the partial pressures of the gases in the mixture
gas law stating that the concentration of gas in a liquid is directly proportional to the solubility and partial pressure of that gas

