84 Disorders of acid-base balance
Learning Objectives
After reading this section you should be able to-
-
Given arterial blood values for PCO2, pH and HCO3–, determine whether a patient is in acidosis or alkalosis and whether the cause of the pH disturbance is metabolic or respiratory.
Normal arterial blood pH is restricted to a very narrow range of 7.35 to 7.45. A person who has a blood pH below 7.35 is considered to be in acidosis (actually, “physiological acidosis,” because blood is not truly acidic until its pH drops below 7.0), and a continuous blood pH below 7.0 can be fatal. Acidosis has several symptoms, including headache and confusion, and the individual can become lethargic and easily fatigued (Figure 83.1). A person who has a blood pH above 7.45 is considered to be in alkalosis, and a pH above 7.8 is fatal. Some symptoms of alkalosis include cognitive impairment (which can progress to unconsciousness), tingling or numbness in the extremities, muscle twitching and spasm, and nausea and vomiting. Both acidosis and alkalosis can be caused by either metabolic or respiratory disorders.
As discussed earlier in this chapter, the concentration of carbonic acid in the blood is dependent on the level of CO2 in the body and the amount of CO2 gas exhaled through the lungs. Thus, the respiratory contribution to acid-base balance is usually discussed in terms of CO2 (rather than of carbonic acid). Remember that a molecule of carbonic acid is lost for every molecule of CO2 exhaled, and a molecule of carbonic acid is formed for every molecule of CO2 retained.
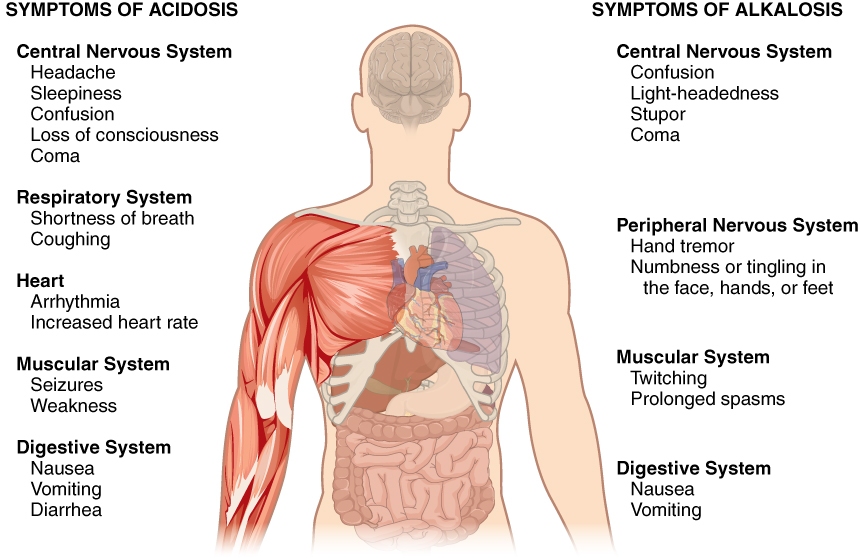
Metabolic Acidosis: Primary Bicarbonate Deficiency
Metabolic acidosis occurs when the blood is too acidic (pH below 7.35) due to too little bicarbonate, a condition called primary bicarbonate deficiency. At the normal pH of 7.40, the ratio of bicarbonate to carbonic acid buffer is 20:1. If a person’s blood pH drops below 7.35, then he or she is in metabolic acidosis. The most common cause of metabolic acidosis is the presence of organic acids or excessive ketones in the blood. Table 83.1 lists some other causes of metabolic acidosis.
| Common Causes of Metabolic Acidosis and Blood Metabolites (Table 83.1) | |
|---|---|
| Cause | Metabolite |
| Diarrhea | Bicarbonate |
| Uremia | Phosphoric and sulfuric acids |
| Diabetic ketoacidosis | Increased ketones |
| Strenuous exercise | Lactate |
| Methanol | Formic acid* |
| Paraldehyde | β-Hydroxybutyric acid* |
| Isopropanol | Propionic acid* |
| Ethylene glycol | Glycolic acid, and some oxalic and formic acids* |
| Salicylate/aspirin | Sulfasalicylic acid (SSA)* |
Metabolic Alkalosis: Primary Bicarbonate Excess
A transient excess of bicarbonate in the blood can follow ingestion of excessive amounts of bicarbonate, citrate, or antacids for conditions such as stomach acid reflux—known as heartburn. Cushing’s disease, which is the chronic hypersecretion of adrenocorticotrophic hormone (ACTH) by the anterior pituitary gland, can cause chronic metabolic alkalosis. The oversecretion of ACTH results in elevated aldosterone levels and an increased loss of potassium by urinary excretion. Other causes of metabolic alkalosis include the loss of hydrochloric acid from the stomach through vomiting, potassium depletion due to the use of diuretics for hypertension, and the excessive use of laxatives.
Respiratory Acidosis: Primary Carbonic Acid/CO2 Excess
Respiratory acidosis occurs when the blood is overly acidic due to an excess of carbonic acid, resulting from too much CO2 in the blood. Respiratory acidosis can result from anything that interferes with respiration, such as pneumonia, emphysema, or congestive heart failure.
Respiratory Alkalosis: Primary Carbonic Acid/CO2 Deficiency
Respiratory alkalosis occurs when the blood is overly alkaline due to a deficiency in carbonic acid and CO2 levels in the blood. This condition usually occurs when too much CO2 is exhaled from the lungs, as occurs in hyperventilation, which is breathing that is deeper or more frequent than normal. An elevated respiratory rate leading to hyperventilation can be due to extreme emotional upset or fear, fever, infections, hypoxia, or abnormally high levels of catecholamines, such as epinephrine and norepinephrine. Surprisingly, aspirin overdose—salicylate toxicity—can result in respiratory alkalosis as the body tries to compensate for initial acidosis.
Compensation Mechanisms
Various compensatory mechanisms exist to maintain blood pH within a narrow range, including buffers, respiration, and renal mechanisms. Although compensatory mechanisms usually work very well, when one of these mechanisms is not working properly (like in kidney failure or respiratory disease), they have their limits. If the pH and bicarbonate to carbonic acid ratio are changed too drastically, the body may not be able to compensate. Moreover, extreme changes in pH can denature proteins. Extensive damage to proteins in this way can result in disruption of normal metabolic processes, serious tissue damage, and ultimately death.
Respiratory Compensation
Respiratory compensation for metabolic acidosis increases the respiratory rate to drive off CO2 and readjust the bicarbonate to carbonic acid ratio to the 20:1 level. This adjustment can occur within minutes. Respiratory compensation for metabolic alkalosis is not as adept as its compensation for acidosis. The normal response of the respiratory system to elevated pH is to increase the amount of CO2 in the blood by decreasing the respiratory rate to conserve CO2. There is a limit to the decrease in respiration, however, that the body can tolerate. Hence, the respiratory route is less efficient at compensating for metabolic alkalosis than for acidosis.
Metabolic Compensation
Metabolic and renal compensation for respiratory diseases that can create acidosis revolves around the conservation of bicarbonate ions. In cases of respiratory acidosis, the kidney increases the conservation of bicarbonate and secretion of H+ through the exchange mechanism discussed earlier. These processes increase the concentration of bicarbonate in the blood, reestablishing the proper relative concentrations of bicarbonate and carbonic acid. In cases of respiratory alkalosis, the kidneys decrease the production of bicarbonate and reabsorb H+ from the tubular fluid. These processes can be limited by the exchange of potassium by the renal cells, which use a K+-H+ exchange mechanism (antiporter).
Diagnosing Acidosis and Alkalosis
Lab tests for pH, CO2 partial pressure (pCO2),and HCO3– can identify acidosis and alkalosis, indicating whether the imbalance is respiratory or metabolic, and the extent to which compensatory mechanisms are working. The blood pH value (Table 83.2) indicates whether the blood is in acidosis, the normal range, or alkalosis. The pCO2 and total HCO3– values aid in determining whether the condition is metabolic or respiratory, and whether the patient has been able to compensate for the problem. Table 83.2 lists the conditions and laboratory results that can be used to classify these conditions. Metabolic acid-base imbalances typically result from kidney disease, and the respiratory system usually responds to compensate.
| Types of Acidosis and Alkalosis (Table 83.2) | |||
|---|---|---|---|
| pH | pCO2 | Total HCO3– | |
| Metabolic acidosis | ↓ | N, then ↓ | ↓ |
| Respiratory acidosis | ↓ | ↑ | N, then ↑ |
| Metabolic alkalosis | ↑ | N, then↑ | ↑ |
| Respiratory alkalosis | ↑ | ↓ | N, then ↓ |
Metabolic acidosis is problematic, as lower-than-normal amounts of bicarbonate are present in the blood. The pCO2 would be normal at first, but if compensation has occurred, it would decrease as the body reestablishes the proper ratio of bicarbonate and carbonic acid/CO2.
Respiratory acidosis is problematic, as excess CO2 is present in the blood. Bicarbonate levels would be normal at first, but if compensation has occurred, they would increase in an attempt to reestablish the proper ratio of bicarbonate and carbonic acid/CO2.
Metabolic alkalosis is problematic, as elevated pH and excess bicarbonate are present. The pCO2 would again be normal at first, but if compensation has occurred, it would increase as the body attempts to reestablish the proper ratios of bicarbonate and carbonic acid/CO2.
Respiratory alkalosis is problematic, as CO2 deficiency is present in the bloodstream. The bicarbonate concentration would be normal at first. When renal compensation occurs, however, the bicarbonate concentration in blood decreases as the kidneys attempt to reestablish the proper ratios of bicarbonate and carbonic acid/CO2 by eliminating more bicarbonate to bring the pH into the physiological range.
Diagnosing Acidosis and Alkalosis: Step-by-Step Approach
To determine whether a patient is in acidosis or alkalosis and identify the cause of the pH disturbance, follow these steps:
- Evaluate Arterial Blood pH:
- pH < 7.35: Indicates acidosis.
- pH > 7.45: Indicates alkalosis.
- Assess Arterial PCO2 and HCO3– Levels:
- PCO2 Normal Range: 35-45 mm Hg
- HCO3– Normal Range: 22-26 mEq/L
- Determine the Primary Cause:
- Respiratory Acidosis: pH < 7.35 and PCO2 > 45 mm Hg (elevated PCO2 indicates CO2 retention).
- Metabolic Acidosis: pH < 7.35 and HCO3– < 22 mEq/L (decreased HCO3– indicates bicarbonate loss or increased acid production).
- Respiratory Alkalosis: pH > 7.45 and PCO2 < 35 mm Hg (decreased PCO2 indicates excessive CO2 exhalation).
- Metabolic Alkalosis: pH > 7.45 and HCO3– > 26 mEq/L (elevated HCO3– indicates excess bicarbonate).
- Check for Compensation:
- Compensated Respiratory Acidosis: Elevated HCO3– in response to increased PCO2.
- Compensated Metabolic Acidosis: Decreased PCO2 in response to low HCO3–.
- Compensated Respiratory Alkalosis: Decreased HCO3– in response to decreased PCO2.
- Compensated Metabolic Alkalosis: Increased PCO2 in response to high HCO3–.
Note on Compensation:
In compensated conditions, pH may return to the normal range (7.35–7.45), but underlying abnormalities in PCO₂ and HCO₃⁻ will still be present. In these cases, the body is partially or fully compensating for the primary disturbance. However, compensation is rarely perfect. If the disturbance is severe or persistent, the pH may remain slightly outside the ideal range.
Adapted from Anatomy & Physiology by Lindsay M. Biga et al, shared under a Creative Commons Attribution-ShareAlike 4.0 International License, chapter 26.
low blood pH
a pH higher than 7.45
a state of acidosis (blood pH below 7.35) caused by too little bicarbonate in the blood
a state of alkalosis (blood pH above 7.45) due to excessive amounts of bicarbonate in the blood
a hormone produced and secreted by the anterior pituitary to stimulate the secretion of corticosteroids involved in the stress response from the adrenal cortex
a state of acidosis (blood pH below 7.35) due to excessive amounts of carbonic acid in the blood, a result of high carbon dioxide
a state of alkalosis (blood pH above 7.45) due to deficient levels of carbonic acid in the blood, resulting from low carbon dioxide
an increased ventilation rate independent of cellular oxygen demands
low blood oxygen levels

