9 Enzymes
Learning Objectives
After reading this section, you should be able to-
- Define enzyme and describe factors that affect enzyme activity.
Proteins Function as Enzymes
If you were trying to type a paper, and every time you hit a key on your laptop there was a delay of six or seven minutes before you got a response, you would probably get a new laptop. In a similar way, without enzymes to catalyze chemical reactions, the human body would be nonfunctional. It functions only because enzymes function.
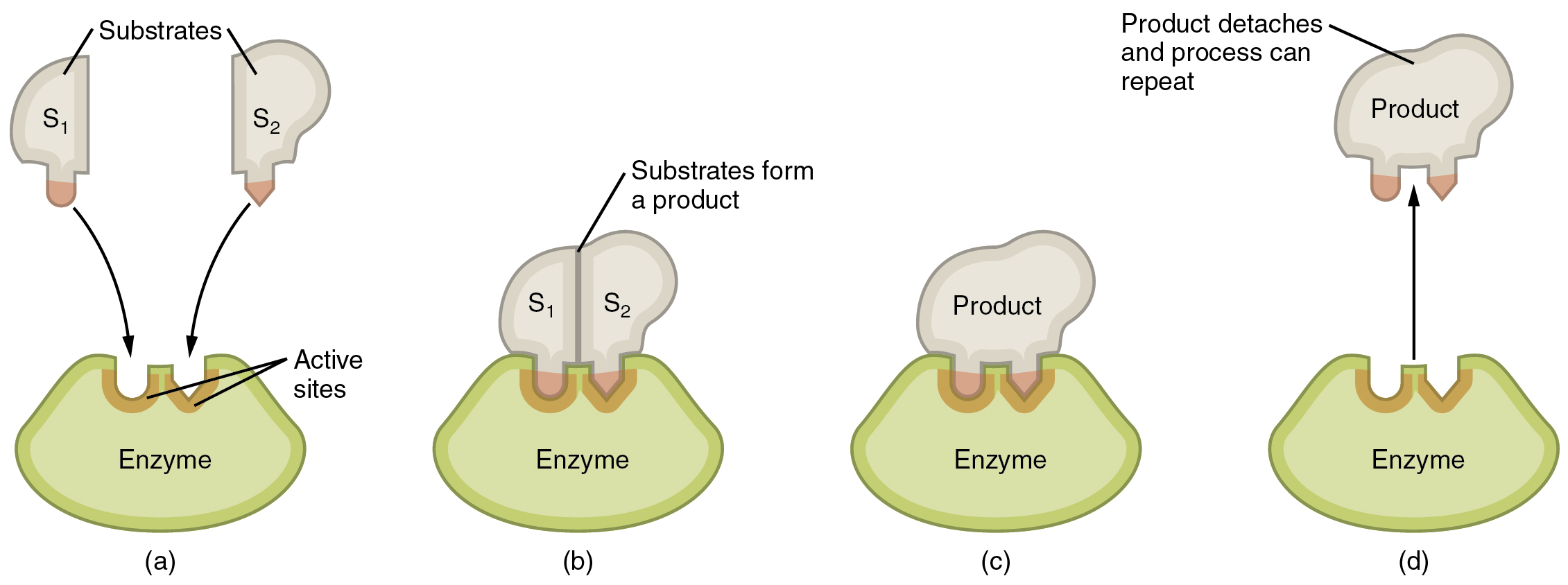
Enzymatic reactions—chemical reactions catalyzed by enzymes—begin when substrates bind to the enzyme. A substrate is a reactant in an enzymatic reaction. This occurs on regions of the enzyme known as active sites (Figure 9.1). Any given enzyme catalyzes just one type of chemical reaction. This characteristic, called specificity, is due to the fact that a substrate with a particular shape and electrical charge can bind only to an active site corresponding to that substrate.
Binding of a substrate produces an enzyme–substrate complex. It is likely that enzymes speed up chemical reactions in part because the enzyme–substrate complex undergoes a set of temporary and reversible changes that cause the substrates to be oriented toward each other in an optimal position to facilitate their interaction. This promotes increased reaction speed. The enzyme then releases the product(s), and resumes its original shape. The enzyme is then free to engage in the process again, and will do so as long as substrate remains.
For two chemicals in nature to react with each other they first have to come into contact. This occurs through random collisions. Since heat helps increase the kinetic energy of atoms, ions, and molecules, it promotes their collision. However, in the body, extremely high heat—such as a very high fever—can damage body cells and be life-threatening. On the other hand, normal body temperature is not high enough to promote the chemical reactions that sustain life. That is where catalysts come in.
In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without undergoing any change itself. You can think of a catalyst as a chemical change agent. They help increase the rate and force at which atoms, ions, and molecules collide, thereby increasing the probability that their valence shell electrons will interact.
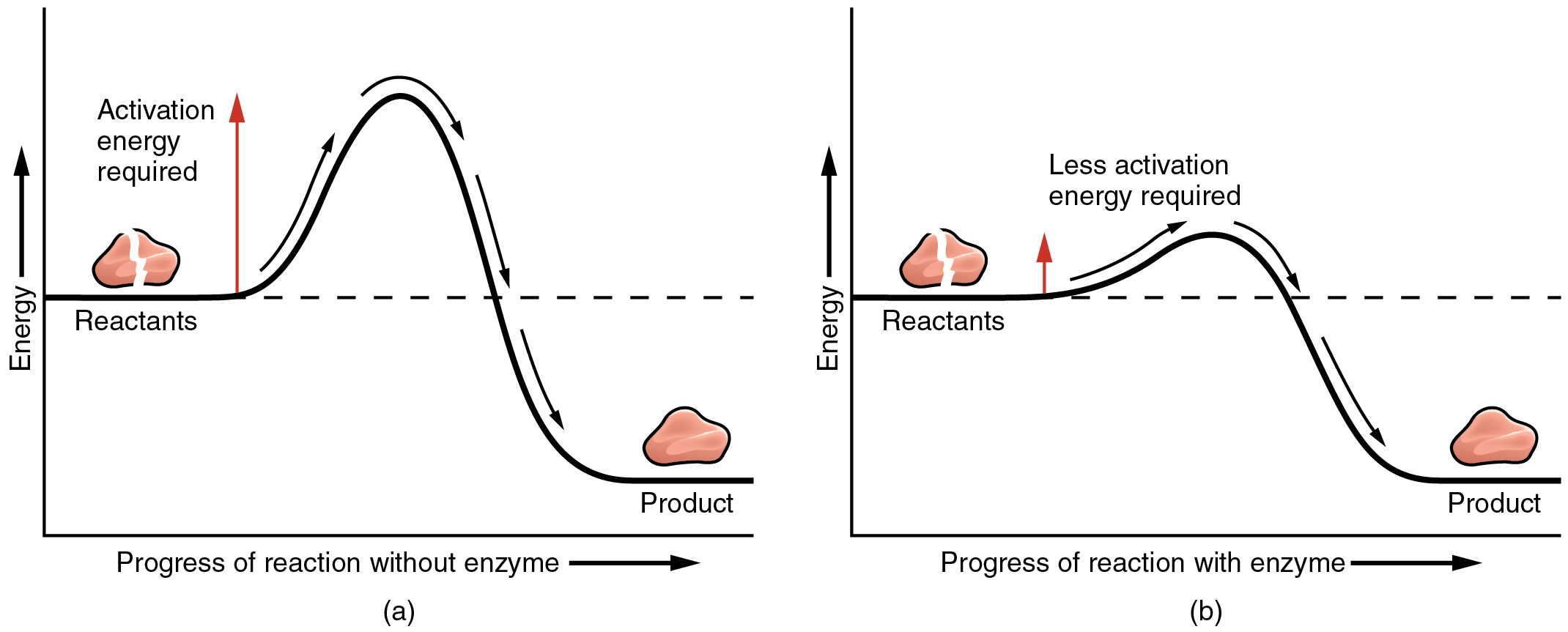
The most important catalysts in the human body are enzymes. An enzyme is a catalyst composed of protein or ribonucleic acid (RNA). Like all catalysts, enzymes work by lowering the level of energy that needs to be invested in a chemical reaction. A chemical reaction’s activation energy is the “threshold” level of energy needed to break the bonds of the reactants. Once those bonds are broken, new arrangements can form. Without an enzyme to act as a catalyst, a much larger investment of energy is needed to ignite a chemical reaction (Figure 9.2).
Factors Affecting Enzyme Activity
Enzymes are subject to several factors that affect their activity. Temperature is one such factor; while normal body temperature optimizes enzyme activity, deviations can have significant effects. A high fever can denature enzymes, rendering them inactive. Conversely, low temperatures can slow down enzymatic reactions. pH levels also play a crucial role. Each enzyme has an optimal pH at which it is most active. Deviations from this optimal pH can lead to a decrease in enzyme activity or even denaturation. For example, the enzyme pepsin works best in the acidic environment of the stomach, whereas trypsin functions optimally in the more alkaline environment of the small intestine. Enzyme and substrate concentrations influence the rate of reactions. Increasing substrate concentration increases the rate of reaction up to a point, after which enzymes become saturated, and the reaction rate levels off. Similarly, increasing enzyme concentration, assuming substrate is in excess, will increase the rate of reaction. Inhibitors and activators are molecules that can decrease or increase enzyme activity, respectively. Competitive inhibitors bind to the active site, blocking substrate access, while non-competitive inhibitors bind to another part of the enzyme, causing a change in its shape and function. Activators can increase enzyme activity by enhancing the enzyme’s ability to bind to substrates. Enzymes are essential for various metabolic processes. Understanding the factors that influence enzyme activity helps in the development of drugs and treatments for diseases involving enzyme malfunction.
Adapted from Anatomy & Physiology by Lindsay M. Biga et al, shared under a Creative Commons Attribution-ShareAlike 4.0 International License, chapter 2.
the molecule that an enzyme reacts with
the site where the enzyme binds; where catalysis takes place
a substance that increases the rate of a reaction without being consumed
a biological catalyst
energy required for a reaction to occur
the process by which the structure of a protein is modified and/or lost by the breaking of bonds within the protein molecule
inhibitor molecules that bind to the active site of an enzyme, blocking a substrate from binding to the enzyme
inhibitor molecule that binds to a distinct part of an enzyme, causing a deviation in the shape and function of that enzyme
molecules that increase enzyme activity by enhancing the ability of the enzyme to bind to substrates

