92 Fertilization and pregnancy
Learning Objectives
After reading this section you should be able to-
- Define fertilization.
- Describe the processes that facilitate fertilization (e.g., sperm capacitation, acrosomal reaction).
- Describe the three phases of fertilization (i.e., corona radiata penetration, zona pellucida penetration, fusion of the oocyte and sperm plasma membranes).
- Describe the formation and function of the placenta and extraembryonic membranes.
- Describe the hormones associated with pregnancy and the effects of these hormones.
Fertilization occurs when a sperm and an oocyte (egg) combine and their nuclei fuse. Because each of these reproductive cells is a haploid cell containing half of the genetic material needed to form a human being, their combination forms a diploid cell. This new single cell, called a zygote, contains all of the genetic material needed to form a human—half from the mother and half from the father.
Transit of Sperm
Fertilization is a numbers game. During ejaculation, hundreds of millions of sperm (spermatozoa) are released into the vagina. Almost immediately, millions of these sperm are overcome by the acidity of the vagina (approximately pH 3.8), and millions more may be blocked from entering the uterus by thick cervical mucus. Of those that do enter, thousands are destroyed by phagocytic uterine leukocytes. Thus, the race into the uterine tubes, which is the most typical site for sperm to encounter the oocyte, is reduced to a few thousand contenders. Their journey—thought to be facilitated by uterine contractions—usually takes anywhere from 30 minutes to 2 hours. If the sperm do not encounter an oocyte immediately, they can survive in the uterine tubes for another 3–5 days. Thus, fertilization can still occur if intercourse takes place a few days before ovulation. In comparison, an oocyte can survive independently for only approximately 24 hours following ovulation. Intercourse more than a day after ovulation will therefore usually not result in fertilization.
During the journey, fluids in the female reproductive tract prepare the sperm for fertilization through a process called capacitation, or priming. The fluids improve the motility of the spermatozoa. They also deplete cholesterol molecules embedded in the membrane of the head of the sperm, thinning the membrane in such a way that will help facilitate the release of the lysosomal (digestive) enzymes needed for the sperm to penetrate the oocyte’s exterior once contact is made. Sperm must undergo the process of capacitation in order to have the “capacity” to fertilize an oocyte. If they reach the oocyte before capacitation is complete, they will be unable to penetrate the oocyte’s thick outer layer of cells.
Contact Between Sperm and Oocyte
Upon ovulation, the oocyte released by the ovary is swept into—and along—the uterine tube. Fertilization must occur in the distal uterine tube because an unfertilized oocyte cannot survive the 72-hour journey to the uterus. As you will recall from your study of oogenesis, this oocyte (specifically a secondary oocyte) is surrounded by two protective layers. The corona radiata is an outer layer of follicular (granulosa) cells that form around a developing oocyte in the ovary and remain with it upon ovulation. The underlying zona pellucida (pellucid = “transparent”) is a transparent, but thick, glycoprotein membrane that surrounds the cell’s plasma membrane.
As it is swept along the distal uterine tube, the oocyte encounters the surviving capacitated sperm, which stream toward it in response to chemical attractants released by the cells of the corona radiata. To reach the oocyte itself, the sperm must penetrate the two protective layers. The sperm first burrow through the cells of the corona radiata. Then, the sperm bind to receptors in the zona pellucida upon contact. This initiates a process called the acrosomal reaction in which the enzyme-filled “cap” of the sperm, called the acrosome, releases its stored digestive enzymes. These enzymes clear a path through the zona pellucida that allows sperm to reach the oocyte. Finally, a single sperm makes contact with sperm-binding receptors on the oocyte’s plasma membrane (Figure 91.1). The plasma membrane of that sperm then fuses with the oocyte’s plasma membrane, and the head and mid-piece of the “winning” sperm enter the oocyte interior.
How do sperm penetrate the corona radiata? Some sperm undergo a spontaneous acrosomal reaction, which is an acrosomal reaction not triggered by contact with the zona pellucida. The digestive enzymes released by this reaction digest the extracellular matrix of the corona radiata. As you can see, the first sperm to reach the oocyte is never the one to fertilize it. Rather, hundreds of sperm cells must undergo the acrosomal reaction, each helping to degrade the corona radiata and zona pellucida until a path is created to allow one sperm to contact and fuse with the plasma membrane of the oocyte. If you consider the loss of millions of sperm between entry into the vagina and degradation of the zona pellucida, you can understand why a low sperm count can cause male infertility.
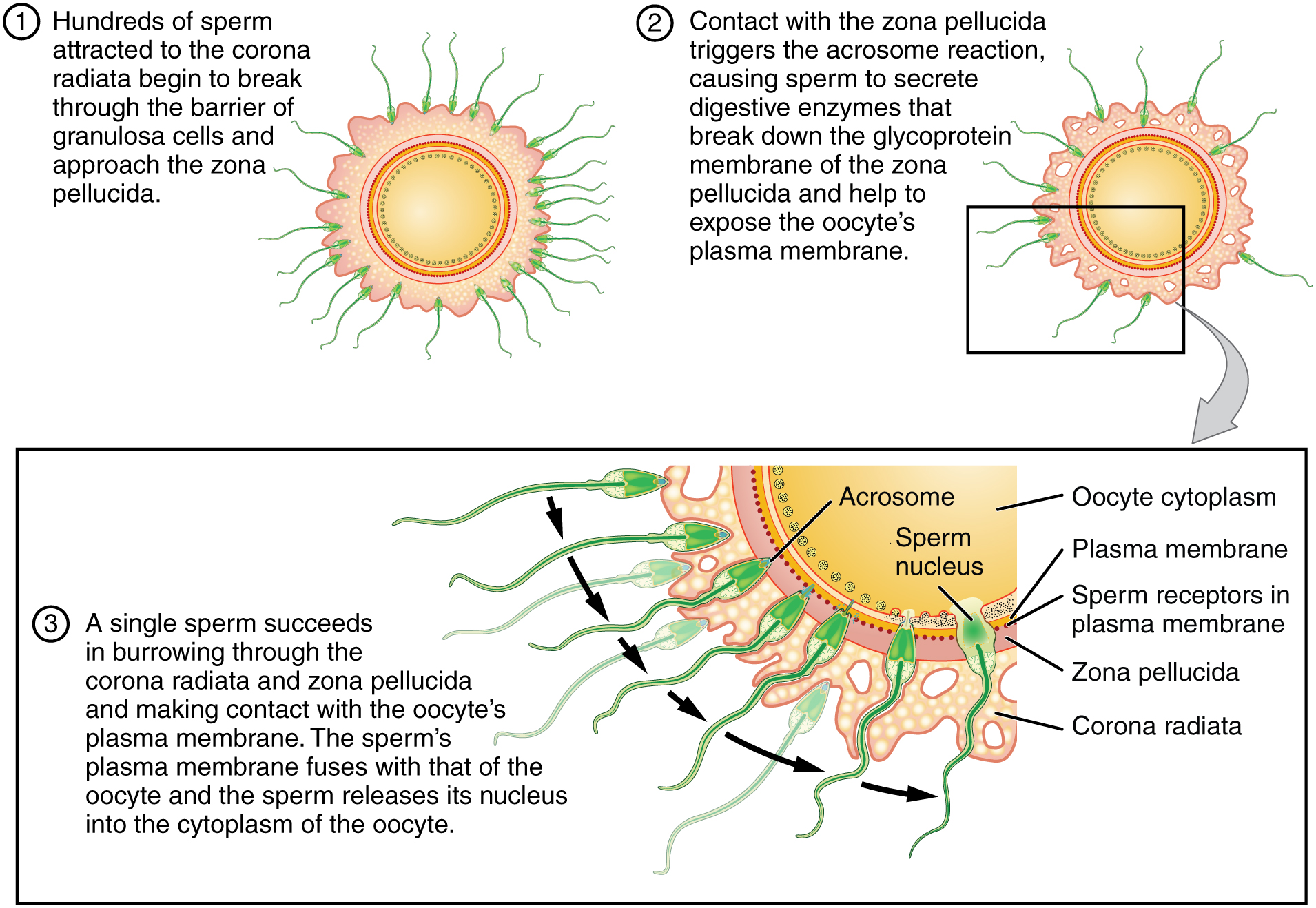
When the first sperm fuses with the oocyte, the oocyte deploys two mechanisms to prevent polyspermy, which is penetration by more than one sperm. This is critical because if more than one sperm were to fertilize the oocyte, the resulting zygote would be a triploid organism with three sets of chromosomes. This is incompatible with life.
The first mechanism is the fast block, which involves a near instantaneous change in sodium ion permeability upon binding of the first sperm, depolarizing the oocyte plasma membrane and preventing the fusion of additional sperm cells. The fast block sets in almost immediately and lasts for about a minute, during which time an influx of calcium ions following sperm penetration triggers the second mechanism, the slow block. In this process, referred to as the cortical reaction, cortical granules sitting immediately below the oocyte plasma membrane fuse with the membrane and release zonal inhibiting proteins and mucopolysaccharides into the space between the plasma membrane and the zona pellucida. Zonal inhibiting proteins cause the release of any other attached sperm and destroy the oocyte’s sperm receptors, thus preventing any more sperm from binding. The mucopolysaccharides then coat the nascent zygote in an impenetrable barrier that, together with hardened zona pellucida, is called a fertilization membrane.
Development of the Placenta
During the first several weeks of development, the cells of the endometrium—referred to as decidual cells—nourish the nascent embryo. During prenatal weeks 4–12, the developing placenta gradually takes over the role of feeding the embryo, and the decidual cells are no longer needed. The mature placenta is composed of tissues derived from the embryo, as well as maternal tissues of the endometrium. The placenta connects to the conceptus via the umbilical cord, which carries deoxygenated blood and wastes from the fetus through two umbilical arteries; nutrients and oxygen are carried from the mother to the fetus through the single umbilical vein. The umbilical cord is surrounded by the amnion, and the spaces within the cord around the blood vessels are filled with Wharton’s jelly, a mucous connective tissue.
The maternal portion of the placenta develops from the deepest layer of the endometrium, the decidua basalis. To form the embryonic portion of the placenta, the syncytiotrophoblast and the underlying cells of the trophoblast (cytotrophoblast cells) begin to proliferate along with a layer of extraembryonic mesoderm cells. These form the chorionic membrane, which envelops the entire conceptus as the chorion. The chorionic membrane forms finger-like structures called chorionic villi that burrow into the endometrium like tree roots, making up the fetal portion of the placenta. The cytotrophoblast cells perforate the chorionic villi, burrow farther into the endometrium, and remodel maternal blood vessels to augment maternal blood flow surrounding the villi. Meanwhile, fetal mesenchymal cells derived from the mesoderm fill the villi and differentiate into blood vessels, including the three umbilical blood vessels that connect the embryo to the developing placenta (Figure 91.2).
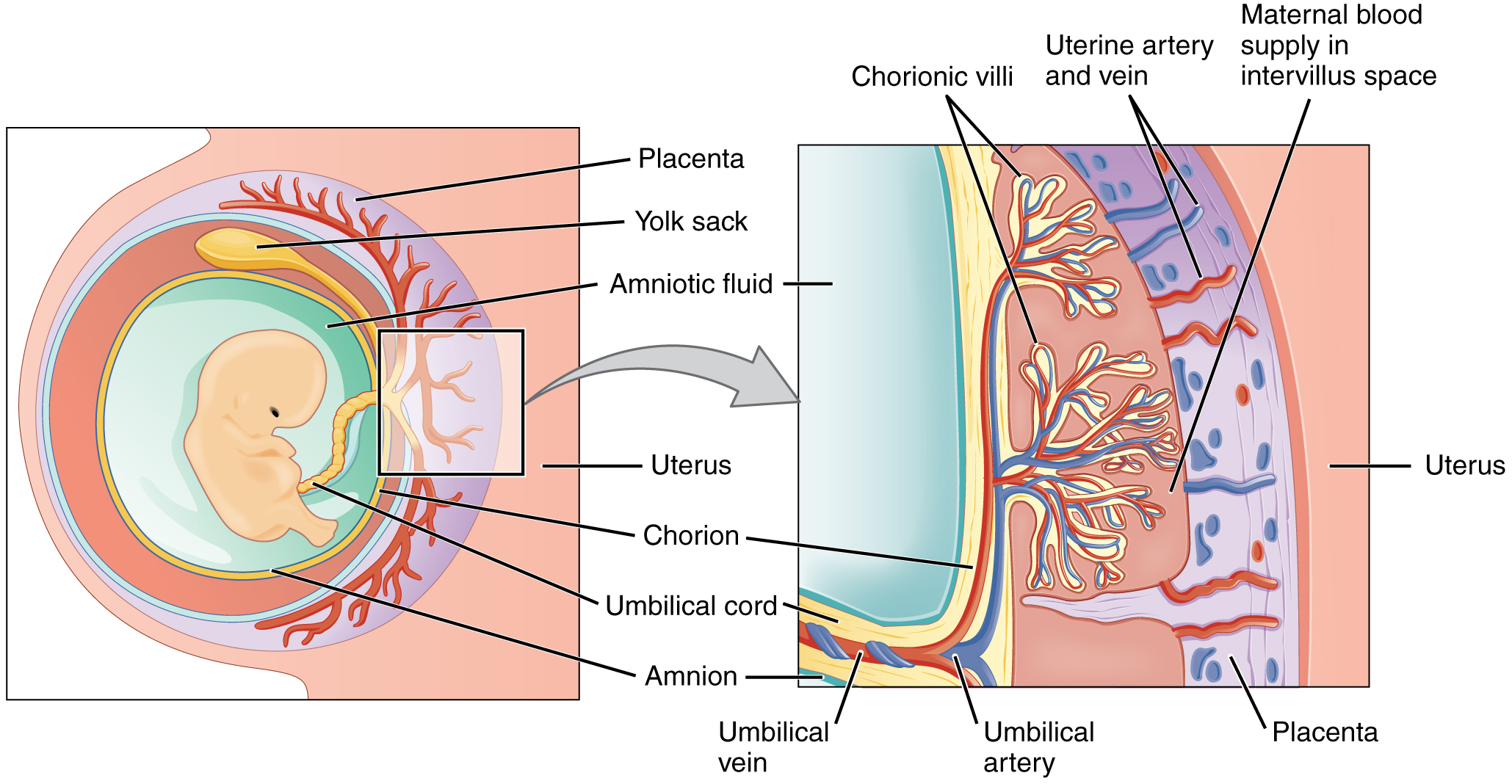
The placenta develops throughout the embryonic period and during the first several weeks of the fetal period; placentation is complete by weeks 14–16. As a fully developed organ, the placenta provides nutrition and excretion, respiration, and endocrine function (Table 91.1 and Figure 91.2). It receives blood from the fetus through the umbilical arteries. Capillaries in the chorionic villi filter fetal wastes out of the blood and return clean, oxygenated blood to the fetus through the umbilical vein. Nutrients and oxygen are transferred from maternal blood surrounding the villi through the capillaries and into the fetal bloodstream. Some substances move across the placenta by simple diffusion. Oxygen, carbon dioxide, and any other lipid-soluble substances take this route. Other substances move across by facilitated diffusion. This includes water-soluble glucose. The fetus has a high demand for amino acids and iron, and those substances are moved across the placenta by active transport.
Maternal and fetal blood does not commingle because blood cells cannot move across the placenta. This separation prevents the mother’s cytotoxic T cells from reaching and subsequently destroying the fetus, which bears “non-self” antigens. Further, it ensures the fetal red blood cells do not enter the mother’s circulation and trigger antibody development (if they carry “non-self” antigens)—at least until the final stages of pregnancy or birth. This is the reason that, even in the absence of preventive treatment, an Rh− mother doesn’t develop antibodies that could cause hemolytic disease in her first Rh+ fetus.
Although blood cells are not exchanged, the chorionic villi provide ample surface area for the two-way exchange of substances between maternal and fetal blood. The rate of exchange increases throughout gestation as the villi become thinner and increasingly branched. The placenta is permeable to lipid-soluble fetotoxic substances: alcohol, nicotine, barbiturates, antibiotics, certain pathogens, and many other substances that can be dangerous or fatal to the developing embryo or fetus. For these reasons, pregnant women should avoid fetotoxic substances. Alcohol consumption by pregnant women, for example, can result in a range of abnormalities referred to as fetal alcohol spectrum disorders (FASD). These include organ and facial malformations, as well as cognitive and behavioral disorders.
| Functions of the Placenta (Table 91.1) | ||
|---|---|---|
| Nutrition and digestion | Respiration | Endocrine function |
|
|
|
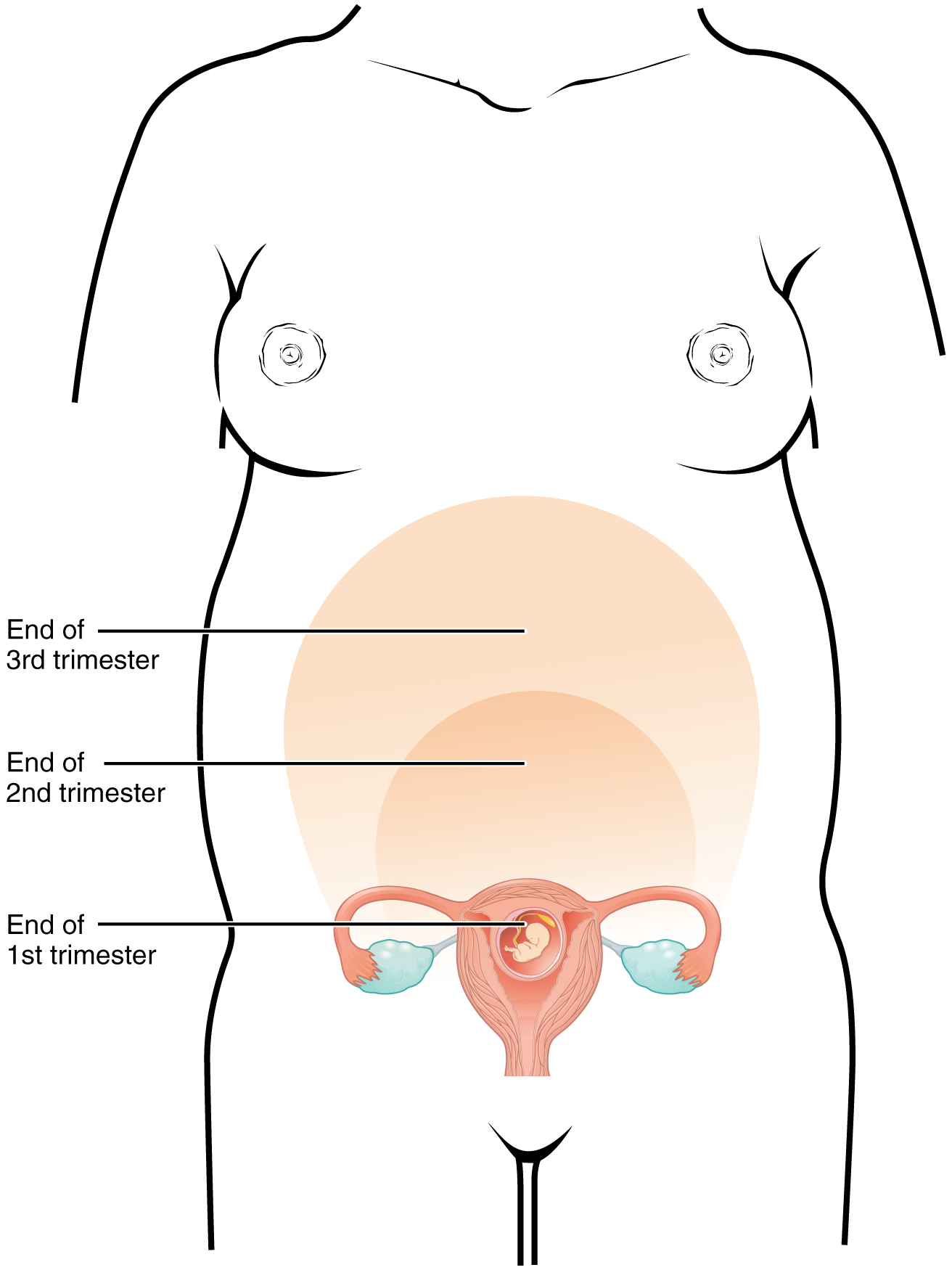
A full-term pregnancy lasts approximately 270 days (approximately 38.5 weeks) from conception to birth. Because it is easier to remember the first day of the last menstrual period (LMP) than to estimate the date of conception, obstetricians set the due date as 284 days (approximately 40.5 weeks) from the LMP. This assumes that conception occurred on day 14 of the woman’s cycle, which is usually a good approximation. The 40 weeks of an average pregnancy are usually discussed in terms of three trimesters, each approximately 13 weeks. During the second and third trimesters, the pre-pregnancy uterus—about the size of a fist—grows dramatically to contain the fetus, causing a number of anatomical changes in the mother (Figure 91.3).
Effects of Hormones
Virtually all of the effects of pregnancy can be attributed in some way to the influence of hormones—particularly estrogens, progesterone, and hCG. During weeks 7–12 from the LMP, the pregnancy hormones are primarily generated by the corpus luteum. Progesterone secreted by the corpus luteum stimulates the production of decidual cells of the endometrium that nourish the blastocyst before placentation. As the placenta develops and the corpus luteum degenerates during weeks 12–17, the placenta gradually takes over as the endocrine organ of pregnancy.
Estrogens
The placenta converts weak androgens secreted by the maternal and fetal adrenal glands to estrogens, which are necessary for pregnancy to progress. Estrogen levels climb throughout the pregnancy, increasing 30-fold by childbirth. Estrogens have the following actions:
- They suppress FSH and LH production, effectively preventing ovulation. (This function is the biological basis of hormonal birth control pills.)
- They induce the growth of fetal tissues and are necessary for the maturation of the fetal lungs and liver.
- They promote fetal viability by regulating progesterone production and triggering fetal synthesis of cortisol, which helps with the maturation of the lungs, liver, and endocrine organs such as the thyroid gland and adrenal gland.
- They stimulate maternal tissue growth, leading to uterine enlargement and mammary duct expansion and branching.
Relaxin
Relaxin, another hormone secreted by the corpus luteum and then by the placenta, helps prepare the mother’s body for childbirth. It increases the elasticity of the symphysis pubic joint and pelvic ligaments, making room for the growing fetus and allowing expansion of the pelvic outlet for childbirth. Relaxin also helps dilate the cervix during labor.
Progesterone
The placenta takes over the synthesis and secretion of progesterone throughout pregnancy as the corpus luteum degenerates. Like estrogen, progesterone suppresses FSH and LH. It also inhibits uterine contractions, protecting the fetus from preterm birth. This hormone decreases in late gestation, allowing uterine contractions to intensify and eventually progress to true labor.
Human Chorionic Gonadotropin (hCG)
The placenta also produces hCG. In addition to promoting survival of the corpus luteum, hCG stimulates the male fetal gonads to secrete testosterone, which is essential for the development of the male reproductive system.
Pituitary hormones
The anterior pituitary enlarges and ramps up its hormone production during pregnancy, raising the levels of thyrotropin, prolactin, and adrenocorticotropic hormone (ACTH). Thyrotropin, in conjunction with placental hormones, increases the production of thyroid hormone, which raises the maternal metabolic rate. This can markedly augment a pregnant woman’s appetite and cause hot flashes. Prolactin stimulates enlargement of the mammary glands in preparation for milk production. ACTH stimulates maternal cortisol secretion, which contributes to fetal protein synthesis. In addition to the pituitary hormones, increased parathyroid levels mobilize calcium from maternal bones for fetal use.
Changes in Organ Systems During Pregnancy
As the woman’s body adapts to pregnancy, characteristic physiologic changes occur. These changes can sometimes prompt symptoms often referred to collectively as the common discomforts of pregnancy.
Digestive and Urinary System Changes
Nausea and vomiting, sometimes triggered by an increased sensitivity to odors, are common during the first few weeks to months of pregnancy. This phenomenon is often referred to as “morning sickness,” although the nausea may persist all day. The source of pregnancy nausea is thought to be the increased circulation of pregnancy-related hormones, specifically circulating estrogen, progesterone, and hCG. Decreased intestinal peristalsis may also contribute to nausea. By about week 12 of pregnancy, nausea typically subsides.
A common gastrointestinal complaint during the later stages of pregnancy is gastric reflux, or heartburn, which results from the upward, constrictive pressure of the growing uterus on the stomach. The same decreased peristalsis that may contribute to nausea in early pregnancy is also thought to be responsible for pregnancy-related constipation as pregnancy progresses.
The downward pressure of the uterus also compresses the urinary bladder, leading to frequent urination. The problem is exacerbated by increased urine production. In addition, the maternal urinary system processes both maternal and fetal wastes, further increasing the total volume of urine.
Circulatory System Changes
Maternal blood volume increases substantially during pregnancy, so that by childbirth it exceeds its preconception volume by 30 percent, or approximately 1–2 liters. The greater blood volume helps to manage the demands of fetal nourishment and fetal waste removal. In conjunction with increased blood volume, the pulse and blood pressure also rise moderately during pregnancy. As the fetus grows, the uterus compresses underlying pelvic blood vessels, hampering venous return from the legs and pelvic region. As a result, many pregnant women develop varicose veins or hemorrhoids.
Respiratory System Changes
During the second half of pregnancy, the respiratory minute volume (volume of gas inhaled or exhaled by the lungs per minute) increases by 50 percent to compensate for the oxygen demands of the fetus and the increased maternal metabolic rate. The growing uterus exerts upward pressure on the diaphragm, decreasing the volume of each inspiration and potentially causing shortness of breath, or dyspnea. During the last several weeks of pregnancy, the pelvis becomes more elastic, and the fetus descends lower in a process called lightening. This typically ameliorates dyspnea.
The respiratory mucosa swell in response to increased blood flow during pregnancy, leading to nasal congestion and nose bleeds, particularly when the weather is cold and dry. Humidifier use and increased fluid intake are often recommended to counteract congestion.
Adapted from Anatomy & Physiology by Lindsay M. Biga et al, shared under a Creative Commons Attribution-ShareAlike 4.0 International License, chapter 28.
the fusion of nuclei between a sperm and oocyte
a diploid cell resulting from the fusion of two haploid gametes; the fertilized ovum
modification of the plasma membrane of sperm once it has entered the female reproductive tract, during which sperm motility is improved by fluids and cholesterol is removed from the head of the sperm, so that penetration and fertilization of the ovum is possible
the release of an oocyte from the ovary
the formation of female gametes (ova, or eggs), occurring in the ovary
outer layer of granulosa (follicular) cells around a developing secondary oocyte in the ovary
the thick acellular membrane secreted by the primary oocyte of a secondary follicle
a chemical reaction that begins when sperm bind to receptors on the zona pellucida of the ovum, during which enzymes are released from the acrosome cap of the sperm that allow the sperm to come into contact with the oocyte
an organelle on the head of mature sperm cells containing enzymes that allow the sperm to penetrate the plasma membrane of an ova and enter the cytoplasm
penetration of an oocyte by more than one sperm
reaction following penetration involving the exocytosis of zonal inhibiting proteins, the contents of cortical granules below the plasma membrane of the oocyte, causing the release of extra sperm and destruction of sperm receptors to prevent polyspermy
a thickened membrane that forms around the zygote (directly after fertilization) to prevent polyspermy
a fetal organ connecting the placenta to the fetus, through which wastes from the fetus are removed through two umbilical arteries and nutrients and oxygen are delivered to the fetus through the umbilical vein
the outermost layer of placenta that is in direct contact with maternal blood
the outermost membrane surrounding the developing embryo that facilitates the exchange of blood and gases between the mother and fetus
finger-like structures formed from the chorionic membrane that provide nutrients from mother to baby; forms the fetal portion of the placenta
layer of mononucleated cells located under the synctiotrophoblast that perforate the chronic vili and augument maternal blood flow
the formation of the placental organ
form of passive transport in which an ion moves directly through the membrane, down its concentration gradient
a form of passive transport in which ions are moved across the membrane with protein interaction
the mass of cells that forms in the ovary, responsible for the production of progesterone and thickening of the uterine lining, following ovulation
groups of stromal and immune cells produced when the corpus luteum secretes progesterone after implantation; responsible for nourishment of the blastocyst and hormone secretion prior to placentation
strong and frequent uterine contractions that cause changes in the cervix, including thinning and dilation
a hormone produced and secreted by the anterior pituitary to regulate the release of hormones from the thyroid gland that increase cellular metabolism
a hormone produced by and secreted from the anterior pituitary that stimulates milk production in the mammary glands of females
a hormone produced and secreted by the anterior pituitary to stimulate the secretion of corticosteroids involved in the stress response from the adrenal cortex

