45 Hemostasis
Jim Davis
Learning Objectives
After studying this section, you should be able to-
- Describe the vascular phase of hemostasis, including the role of endothelial cells.
- Describe the role of platelets in hemostasis and the steps involved in the formation of the platelet plug.
- Describe the basic steps of coagulation resulting in the formation of the insoluble fibrin clot.
- Differentiate among the intrinsic (contact activation), extrinsic (cell injury), and common pathways of the coagulation cascade.
- Explain how the positive feedback loops in the platelet and coagulation phases promote hemostasis.
- Explain the role of vitamin K in blood clotting.
- Describe the process of fibrinolysis, including the roles of plasminogen, tissue plasminogen activator, and plasmin.
Platelets are key players in hemostasis, the process by which the body seals a ruptured blood vessel and prevents further loss of blood. Although rupture of larger vessels usually requires medical intervention, hemostasis is quite effective in dealing with small, simple wounds. There are three steps to the process: vascular spasm, the formation of a platelet plug, and coagulation (blood clotting). Failure of any of these steps will result in hemorrhage—excessive bleeding.
Vascular Spasm
When a vessel is severed or punctured, or when the wall of a vessel is damaged, vascular spasm occurs.This vascular spasm occurs to reduce pressure to the damaged area of the vessel, and minimize blood loss. This process is similar to a plumber shutting off the water prior to repairing a broken pipe. The vascular spasm response is believed to be triggered by several chemicals called endothelins that are released by vessel-lining cells and by pain receptors in response to vessel injury. This phenomenon typically lasts for up to 30 minutes, although it can last for several hours.
Formation of the Platelet Plug
In the second step, platelets, which normally float free in the plasma, encounter exposed connective tissue found inside the damaged vessel. This causes platelets to clump together, become spiked and sticky, and bind to the exposed collagen and endothelial lining. This process is assisted by a glycoprotein in the blood plasma called von Willebrand factor (vWf), which helps stabilize the growing platelet plug. As platelets collect, they simultaneously release chemicals from their granules into the plasma that further contribute to hemostasis. Among the substances released by the platelets are:
- Adenosine diphosphate (ADP) – helps additional platelets to adhere to the injury site, reinforcing and expanding the platelet plug
- Serotonin – maintains vasoconstriction
- Prostaglandins and phospholipids – maintain vasoconstriction and help to activate further clotting chemicals
A platelet plug temporarily seals a small opening in a blood vessel, which allows the body time to make more sophisticated and durable repairs.
Coagulation
These sophisticated and durable repairs made beyond the plug formation are collectively called coagulation, the formation of a blood clot. The process is sometimes characterized as a cascade, because one event prompts the next as in a multi-level waterfall. The result is the production of a gelatinous but robust clot made up of a mesh of an insoluble protein called fibrin, which is derived from a plasma protein called fibrinogen. This mesh traps platelets and blood cells (Figure 44.1).
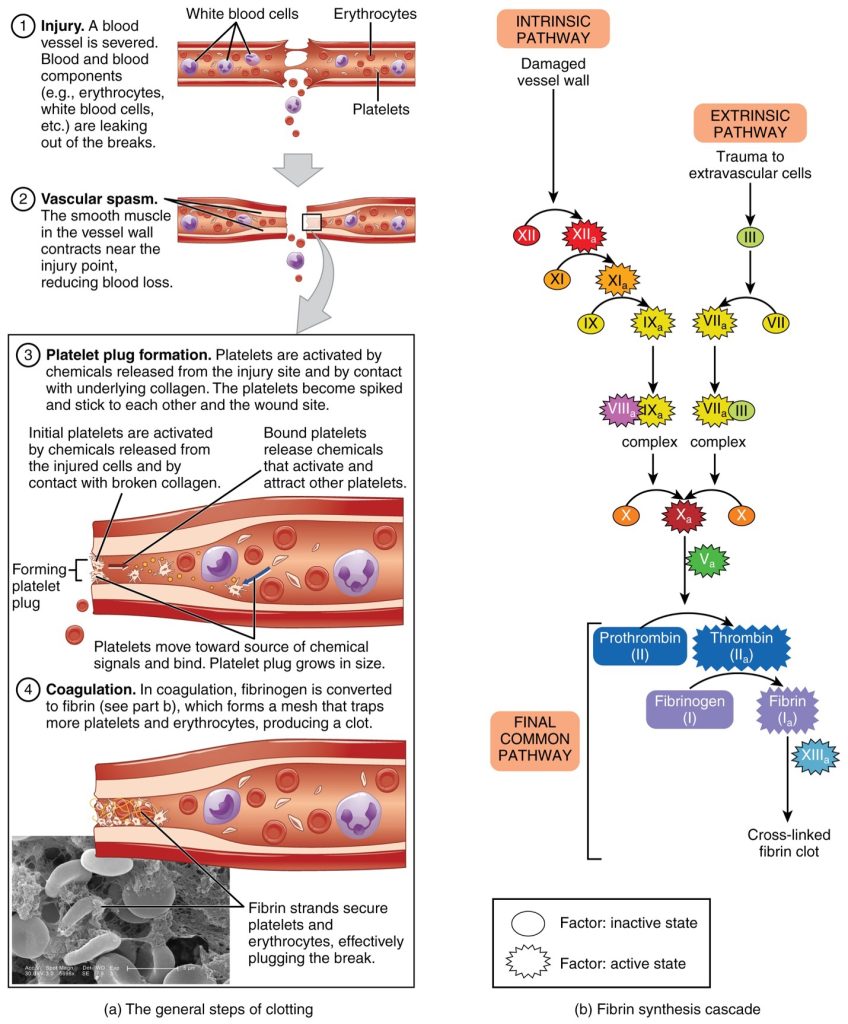
Clotting Factors Involved in Coagulation
In the coagulation cascade, chemicals called clotting factors (or coagulation factors) prompt reactions that activate more coagulation factors. The process is complex, but is initiated along two basic pathways:
- The extrinsic pathway, which normally is triggered by trauma.
- The intrinsic pathway, which begins in the bloodstream and is triggered by internal damage to the wall of the vessel.
Both of these merge into a third pathway, referred to as the common pathway. All three pathways are dependent upon the 12 known clotting factors, including Ca2+ and vitamin K. Clotting factors are secreted primarily by the liver and the platelets. The liver requires vitamin K to produce many of them. Vitamin K is essential because it enables the liver to synthesize several clotting factors, including factors II (prothrombin), VII, IX, and X. Without adequate vitamin K, these factors cannot function properly, leading to impaired clotting and an increased risk of bleeding. Conditions like vitamin K deficiency or the use of certain medications (e.g., warfarin) that block vitamin K activity can result in serious bleeding disorders.The calcium ion, considered factor IV, is derived from the diet and from the breakdown of bone.
The 12 clotting factors are numbered I through XII according to the order of their discovery, not the order they are activated.
Extrinsic Pathway
The quicker responding and more direct extrinsic pathway begins when damage occurs to the surrounding tissues, such as in a traumatic injury. Upon contact with blood plasma, the damaged extravascular cells, which are extrinsic to the bloodstream, release factor III. Sequentially, Ca2+ then factor VI, which is activated by factor III, are added, forming an enzyme complex. This enzyme complex leads to activation of factor X, which activates the common pathway discussed below. The events in the extrinsic pathway are completed in a matter of seconds.
Intrinsic Pathway
The intrinsic pathway is longer and more complex. In this case, the factors involved are intrinsic to (present within) the bloodstream. The pathway is most often initiated when factor XII comes into contact with foreign materials. Within the body, factor XII is typically activated when it encounters negatively charged molecules, such as inorganic polymers and phosphate produced earlier in the series of intrinsic pathway reactions. Factor XII sets off a series of reactions that in turn activates factor X then factor I. In the meantime, chemicals released by the platelets increase the rate of these activation reactions. Finally, factor VIII from the platelets and endothelial cells combines with factor IX to form an enzyme complex that also activates factor X, leading to the common pathway. The events in the intrinsic pathway are completed in a few minutes.
Common Pathway
Both the intrinsic and extrinsic pathways lead to the common pathway, in which fibrin is produced to seal off the vessel. Once factor X has been activated the enzyme prothrombinase converts factor II into the active enzyme thrombin. Then, thrombin converts factor I, the soluble fibrinogen, into the insoluble fibrin protein strands. Factor XIII then stabilizes the fibrin clot.
The coagulation cascade and platelet plug formation both rely on positive feedback loops to ensure rapid and efficient clotting. For example, activated platelets release chemicals like ADP that recruit and activate even more platelets, amplifying the formation of the platelet plug. Similarly, thrombin generated in the common pathway not only converts fibrinogen to fibrin but also accelerates the activation of upstream clotting factors, further boosting clot formation. These feedback loops ensure that once clotting is initiated, it proceeds quickly enough to prevent excessive blood loss.
Fibrinolysis
The stabilized clot is acted upon by contractile proteins within the platelets. As these proteins contract, they pull on the fibrin threads, bringing the edges of the clot more tightly together, somewhat as we do when tightening loose shoelaces. This process also wrings a small amount of fluid (serum) out of the clot, which is blood plasma without its clotting factors.
To restore normal blood flow as the vessel heals, the clot must eventually be removed. Fibrinolysis the gradual degradation of the clot (Figure 44.2). Again, there is a fairly complicated series of reactions that involves factor XII and protein-catabolizing enzymes. During this process, the inactive protein plasminogen is converted into the active plasmin, which gradually breaks down the fibrin of the clot. Additionally, bradykinin, a vasodilator, is released, reversing the effects of the serotonin and prostaglandins from the platelets. This allows the smooth muscle in the walls of the vessels to relax and helps to restore the circulation.
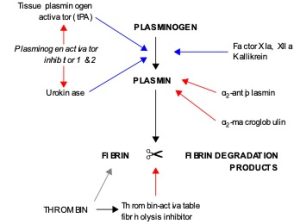
Summary
Hemostasis is the physiological process by which bleeding ceases. Hemostasis involves three basic steps: vascular spasm, the formation of a platelet plug, and coagulation, in which clotting factors promote the formation of a fibrin clot. Fibrinolysis is the process in which a clot is degraded in a healing vessel. Anticoagulants are substances that oppose coagulation. They are important in limiting the extent and duration of clotting. Inadequate clotting can result from too few platelets, or inadequate production of clotting factors, for instance, in the genetic disorder hemophilia. Excessive clotting, called thrombosis, can be caused by excessive numbers of platelets. A thrombus is a collection of fibrin, platelets, and erythrocytes that has accumulated along the lining of a blood vessel, whereas an embolus is a thrombus that has broken free from the vessel wall and is circulating in the bloodstream.
Adapted from Anatomy & Physiology by Lindsay M. Biga et al, shared under a Creative Commons Attribution-ShareAlike 4.0 International License, chapter 18; “Fibrinolysis”; Wikimedia (CC BY-SA 3.0)
The process by which the body seals a ruptured blood vessel and prevents further loss of blood
the first step of hemostasis in which pressure at the area of vessel damage is reduced, and the vessel vasoconstricts to minimize blood loss
chemicals released by vessel-lining cells and nociceptors in response to vessel injury
the second step of hemostasis, in which platelets become sticky and bind to the exposed collagen of a damaged vessel in order to temporarily seal the opening in the vessel and further minimize blood loss
the last step of hemostasis, in which a blood clot is formed
an insoluble protein formed from fibrinogen during the blood clotting cascade, and which forms the meshwork of the blood clot
the blood clotting pathway consisting of coagulation factors found within the damaged vessel
the blood clotting pathway consisting of coagulation factors found within the plasma
the final blood clotting pathway, activated by factor X, in which fibrin is produced to seal off a damaged vessel
gradual degradation of a blood clot
the active form of the enzyme plasminogen that degrades the fibrin meshwork of a blood clot
substances that oppose coagulation

