86 Spermatogenesis
Learning Objectives
After reading this section, you should be able to-
-
Define the processes of spermatogenesis and spermiogenesis.
-
Describe the stages of spermatogenesis in the seminiferous tubule, including the roles of the nurse (sustentacular, Sertoli) cells and interstitial cells (of Leydig).
-
Describe endocrine regulation of spermatogenesis
Sustentacuar Cells
Surrounding all stages of the developing sperm cells are elongate, branching Sertoli cells. Sertoli cells are a type of supporting cell called a sustentacular cell, or sustentocyte, that are typically found in epithelial tissue. Sertoli cells secrete signaling molecules that promote sperm production and can control whether germ cells live or die. They extend physically around the germ cells from the peripheral basement membrane of the seminiferous tubules to the lumen. Tight junctions between these sustentacular cells create the blood–testis barrier, which keeps bloodborne substances from reaching the germ cells and, at the same time, keeps surface antigens on developing germ cells from escaping into the bloodstream and prompting an autoimmune response.
Additional Detail on Sertoli Cell Functions
Sertoli cells, in addition to their crucial role as sustentacular cells, play a multifaceted role in supporting the development of germ cells. These specialized cells extend from the basement membrane to the lumen of the seminiferous tubules, creating a physical environment that nurtures and guides spermatogenesis. Beyond providing structural support, Sertoli cells contribute significantly to the nutritional and metabolic well-being of developing germ cells.
Sertoli cells are equipped with the ability to produce signaling molecules and growth factors that stimulate and regulate sperm production. They actively secrete nutrients, including glucose and amino acids, essential for the energy-intensive process of spermatogenesis. This nutritional support ensures the optimal development and maturation of germ cells, promoting the formation of healthy sperm.
Moreover, Sertoli cells are involved in the clearance of excess cytoplasm during spermiogenesis, a process known as residual body phagocytosis. This crucial function helps shape the mature sperm by removing unnecessary cellular components, contributing to the formation of streamlined and functional spermatozoa. FSH also stimulates Sertoli cells to secrete androgen-binding protein (ABP), which binds and concentrates testosterone in the seminiferous tubule lumen, maintaining high local androgen levels essential for spermatogenesis.
Germ Cells
The least mature cells, the spermatogonia (singular = spermatogonium), line the basement membrane inside the tubule. Spermatogonia are the stem cells of the testis, which means that they are still able to differentiate into a variety of different cell types throughout adulthood. Spermatogonia divide to produce primary and secondary spermatocytes, then spermatids, which finally produce formed sperm. The process that begins with spermatogonia and concludes with the production of sperm is called spermatogenesis.
Spermatogenesis
As previously noted, spermatogenesis occurs in the seminiferous tubules that form the bulk of each testis (see Figure 85.1). The process begins at puberty, after which time sperm are produced constantly throughout a man’s life. One production cycle, from spermatogonia through formed sperm, takes approximately 64 days. A new cycle starts approximately every 16 days, although this timing is not synchronous across the seminiferous tubules. Sperm counts—the total number of sperm a man produces—slowly decline after age 35, and some studies suggest that smoking can lower sperm counts irrespective of age.
The process of spermatogenesis begins with mitosis of the diploid spermatogonia (Figure 85.1). Because these cells are diploid (2n), they each have a complete copy of the father’s genetic material, or 46 chromosomes. However, mature gametes are haploid (1n), containing 23 chromosomes—meaning that daughter cells of spermatogonia must undergo a second cellular division through the process of meiosis.
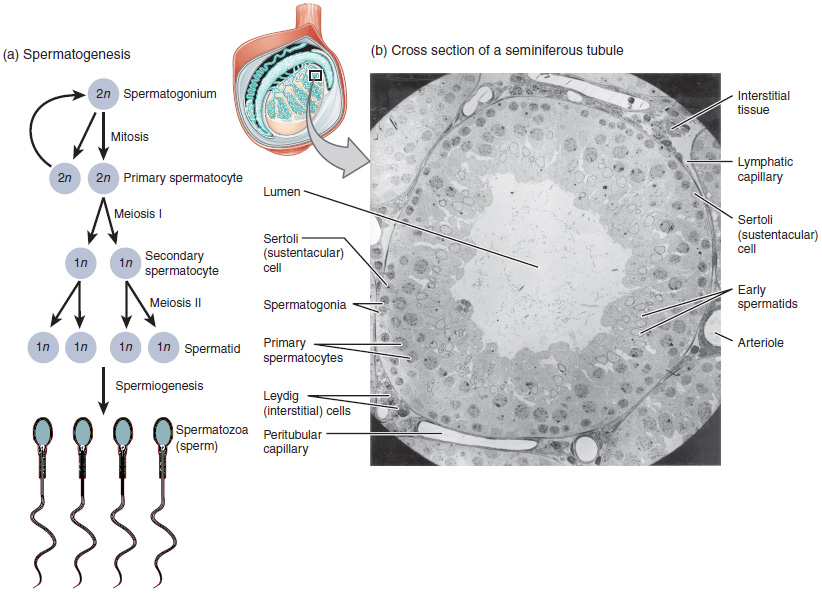
Two identical diploid cells result from spermatogonia mitosis. One of these cells remains a spermatogonium, and the other becomes a primary spermatocyte in the next stage of spermatogenesis. As in mitosis, DNA is replicated in a primary spermatocyte, before it undergoes a cell division called meiosis I. During meiosis I each of the 23 pairs of chromosomes separates. This results in two cells, called secondary spermatocytes, each with only half the number of chromosomes. Now a second round of cell division (meiosis II) occurs in both of the secondary spermatocytes.
During meiosis II, each of the 23 replicated chromosomes divides, similar to what happens during mitosis. Thus, meiosis results in separating the chromosome pairs. This second meiotic division results in a total of four cells with only half of the number of chromosomes. Each of these new cells is a spermatid. Although haploid, early spermatids look very similar to cells in the earlier stages of spermatogenesis, with a round shape, central nucleus, and large amount of cytoplasm. A process called spermiogenesis transforms these early spermatids, reducing the cytoplasm, and beginning the formation of the parts of a true sperm. The fifth stage of germ cell formation—spermatozoa, or formed sperm—is the end result of this process, which occurs in the portion of the tubule nearest the lumen. Eventually, the sperm are released into the lumen and are moved along a series of ducts in the testis toward a structure called the epididymis for the next step of sperm maturation.
Structure of Formed Sperm
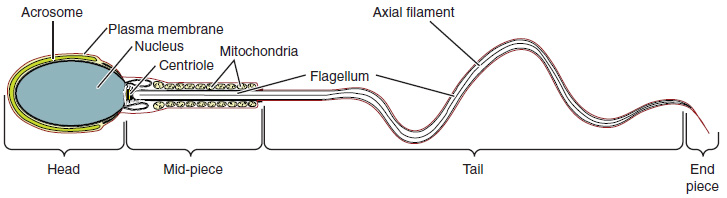
Sperm are smaller than most cells in the body, with a distinctive head, mid-piece, and tail region. The head contains the compact haploid nucleus and an acrosome filled with lysosomal enzymes crucial for fertilization. The mid-piece houses tightly packed mitochondria that provide ATP for the flagellum, enabling sperm movement. The tail, formed from the axial filament, propels the sperm cell. (Figure 85.2).
Sperm Transport
To fertilize an egg, sperm must be moved from the seminiferous tubules in the testes, through the epididymis, and—later during ejaculation—along the length of the penis and out into the female reproductive tract.
Testosterone
Testosterone, an androgen, is a steroid hormone produced by Leydig cells. The alternate term for Leydig cells, interstitial cells, reflects their location between the seminiferous tubules in the testes. In male embryos, Leydig cells begin secreting testosterone by the seventh week of development, with peak concentrations reached in the second trimester. This early release of testosterone results in the anatomical differentiation of the male sexual organs. In childhood, testosterone concentrations are low. They increase during puberty, activating characteristic physical changes and initiating spermatogenesis.
Functions of Testosterone
The continued presence of testosterone is necessary to keep the male reproductive system working properly, and Leydig cells produce approximately 6 to 7 mg of testosterone per day. Testicular steroidogenesis (the manufacture of androgens, including testosterone) results in testosterone concentrations that are 100 times higher in the testes than in the circulation. Maintaining these normal concentrations of testosterone promotes spermatogenesis, whereas low levels of testosterone can lead to infertility. In addition to intratesticular secretion, testosterone is also released into the systemic circulation and plays an important role in muscle development, bone growth, the development of secondary sex characteristics, and maintaining libido (sex drive) in both males and females. In females, the ovaries secrete small amounts of testosterone, although most is converted to estradiol. A small amount of testosterone is also secreted by the adrenal glands in both sexes.
Control of Testosterone
The regulation of testosterone concentrations throughout the body is critical for male reproductive function. The intricate interplay between the endocrine system and the reproductive system is shown in Figure 85.3.
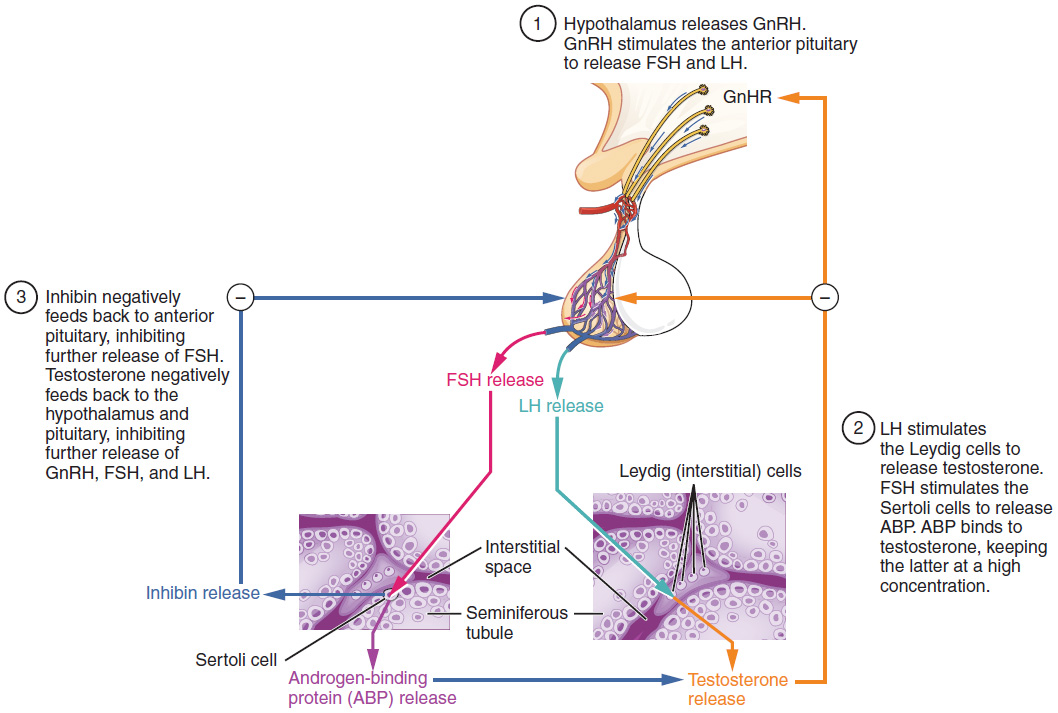
The regulation of testosterone production by Leydig cells begins outside of the testes. The hypothalamus and the pituitary gland in the brain integrate external and internal signals to control testosterone synthesis and secretion. The regulation begins in the hypothalamus. Pulsatile release of a hormone called gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the endocrine release of hormones from the anterior pituitary gland. Binding of GnRH to its receptors on the anterior pituitary stimulates the release of the two gonadotropins: luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These two hormones are critical for reproductive function in both men and women. In men, FSH binds predominantly to the Sertoli cells within the seminiferous tubules to promote spermatogenesis. FSH also stimulates the Sertoli cells to produce hormones called inhibins, which function to inhibit FSH release from the pituitary, thus reducing testosterone secretion. These polypeptide hormones correlate directly with Sertoli cell function and sperm number; inhibin B can be used as a marker of spermatogenic activity. In men, LH binds to receptors on Leydig cells in the testes and upregulates the production of testosterone.
A negative feedback loop predominantly controls the synthesis and secretion of both FSH and LH. Low blood concentrations of testosterone stimulate the hypothalamic release of GnRH. GnRH then stimulates the anterior pituitary to secrete LH into the bloodstream. In the testis, LH binds to LH-specific receptors on Leydig cells and stimulates the release of testosterone. When concentrations of testosterone in the blood reach a critical threshold, testosterone itself will bind to androgen receptors on both the hypothalamus and the anterior pituitary, inhibiting the synthesis and secretion of GnRH and LH, respectively. When the blood concentrations of testosterone once again decline, testosterone no longer interacts with the receptors to the same degree and GnRH and LH are once again secreted, stimulating more testosterone production. This same process occurs with FSH and inhibin to control spermatogenesis.
Adapted from Anatomy & Physiology by Lindsay M. Biga et al, shared under a Creative Commons Attribution-ShareAlike 4.0 International License, chapter 27.
cells within the seminiferous tubules of the testes responsible for supporting developing sperm cells
mature sperm cells
stem cells located in the testes that may mature to become spermatozoa; undifferentiated male germ cells
the formation of mature sperm cells from spermatogonia, taking place in the seminiferous tubules of the testes and beginning at the onset of male puberty, with one cycle lasting 64 days
the process by which a single parent cell divides to form two genetically identical (diploid) daughter cells
the process by which a single cell divides twice to produce four genetically different (haploid) daughter cells
a diploid cell derived from the mitotic division of a spermatogonium
haploid cells derived from the first meiotic division of a primary spermatocyte
haploid cells derived from secondary spermatocytes, the result of the second meiotic division; immature sperm cells that will become spermatozoa
the final stage of spermatogenesis during which spermatids mature into spermatozoa
an organelle on the head of mature sperm cells containing enzymes that allow the sperm to penetrate the plasma membrane of an ova and enter the cytoplasm
a steroid hormone produced by Leydig/interstitial cells between the seminiferous tubules of the testes, responsible for anatomical differentiation of male sex organs in utero and, after puberty, for the activation of physical male sex characteristics and spermatogenesis
cells located between the seminiferous tubules in the testes responsible for secreting testosterone, beginning in utero for anatomical differentiation of male sex organs, and during puberty to activate male physical sex characteristics and spermatogenesis
a hormone produced and secreted by the anterior pituitary that causes the production of reproductive hormones in the gonads of males and females, and ovulation in females
a hormone produced by and released from the anterior pituitary that stimulates the production and maturation of gametes in males and females, and the growth of ovarian follicles in females

