10 Transport mechanisms
Learning Objectives
After reading this section, you should be able to-
- Compare and contrast intracellular and extra cellular fluid with respect to chemical composition and location
- Compare and contrast simple diffusion across membranes and facilitated diffusion with respect to their mechanisms, the type of material being moved, and the energy source for their movement
- Compare and contrast facilitated diffusion and active transport with respect to their mechanisms, the type of material being moved, and the energy source for their movement
- Define osmosis and explain how it differs from simple diffusion across membranes
- Compare and contrast osmolarity and tonicity of solutions.
- Describe the effects of hypertonic, isotonic, and hypotonic solutions on cells.
Transport Across the Cell Membrane
One of the great wonders of the cell membrane is its ability to regulate the concentration of substances inside the cell. These substances include ions such as Ca2+, Na+, K+, and Cl–, nutrients including sugars, fatty acids, and amino acids, and waste products, particularly carbon dioxide (CO2), which must leave the cell.
The membrane’s lipid bilayer structure provides the first level of control. The phospholipids are tightly packed together, and the membrane has a hydrophobic interior. This structure causes the membrane to be selectively permeable. A membrane that has selective permeability allows only substances meeting certain criteria to pass through it unaided. In the case of the cell membrane, only relatively small, nonpolar materials can move through the lipid bilayer (remember, the lipid tails of the membrane are nonpolar). Some examples of these are other lipids, oxygen and carbon dioxide gases, and alcohol. However, water-soluble materials—like glucose, amino acids, and electrolytes—need some assistance to cross the membrane because they are repelled by the hydrophobic tails of the phospholipid bilayer. All substances that move through the membrane do so by one of two general methods, which are categorized based on whether or not energy is required. Passive transport is the movement of substances across the membrane without the expenditure of cellular energy. In contrast, active transport is the movement of substances across the membrane using energy from adenosine triphosphate (ATP).
Passive Transport
In order to understand how substances move passively across a cell membrane, it is necessary to understand concentration gradients and diffusion. A concentration gradient is the difference in concentration of a substance across a space. Molecules (or ions) will spread/diffuse from where they are more concentrated to where they are less concentrated until they are equally distributed in that space. (When molecules move in this way, they are said to move down their concentration gradient, from high concentration to low concentration.) Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. A couple of common examples will help to illustrate this concept. Imagine being inside a closed room. If a bottle of perfume were sprayed, the scent molecules would naturally diffuse from the spot where they left the bottle to all corners of the room, and this diffusion would go on until the molecules were equally distributed in the room. Another example is a spoonful of sugar placed in a cup of tea. Eventually the sugar will diffuse throughout the tea until no concentration gradient remains. In both cases, if the room is warmer or the tea hotter, diffusion occurs even faster as the molecules are bumping into each other and spreading out faster than at cooler temperatures.
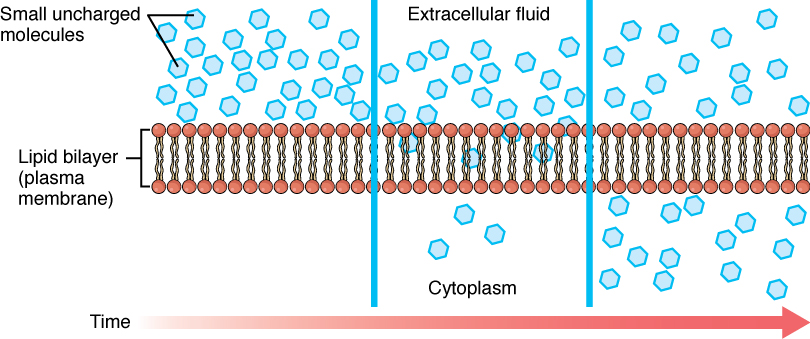
Whenever a substance exists in greater concentration on one side of a semipermeable membrane, such as cell membranes, any substance that can move down its concentration gradient across the membrane will do so. If the substances can move across the cell membrane without the cell expending energy, the movement of molecules is called passive transport. Consider substances that can easily diffuse through the lipid bilayer of the cell membrane, such as the gases oxygen (O2) and carbon dioxide (CO2). These small, fat soluble gasses and other small lipid soluble molecules can dissolve in the membrane and enter or exit the cell following their concentration gradient. This mechanism of molecules moving across a cell membrane from the side where they are more concentrated to the side where they are less concentrated is a form of passive transport called simple diffusion. O2 generally diffuses into cells because it is more concentrated outside of them, and CO2 typically diffuses out of cells because it is more concentrated inside of them.
Before moving on, it is important to realize that the concentration gradients for oxygen and carbon dioxide will always exist across a living cell and never reach equal distribution. This is because cells rapidly use up oxygen during metabolism and so, there is typically a lower concentration of O2 inside the cell than outside. As a result, oxygen will diffuse from outside the cell directly through the lipid bilayer of the membrane and into the cytoplasm within the cell. On the other hand, because cells produce CO2 as a byproduct of metabolism, CO2 concentrations rise within the cytoplasm; therefore, CO2 will move from the cell through the lipid bilayer and into the extracellular fluid, where its concentration is lower (Figure 10.1).
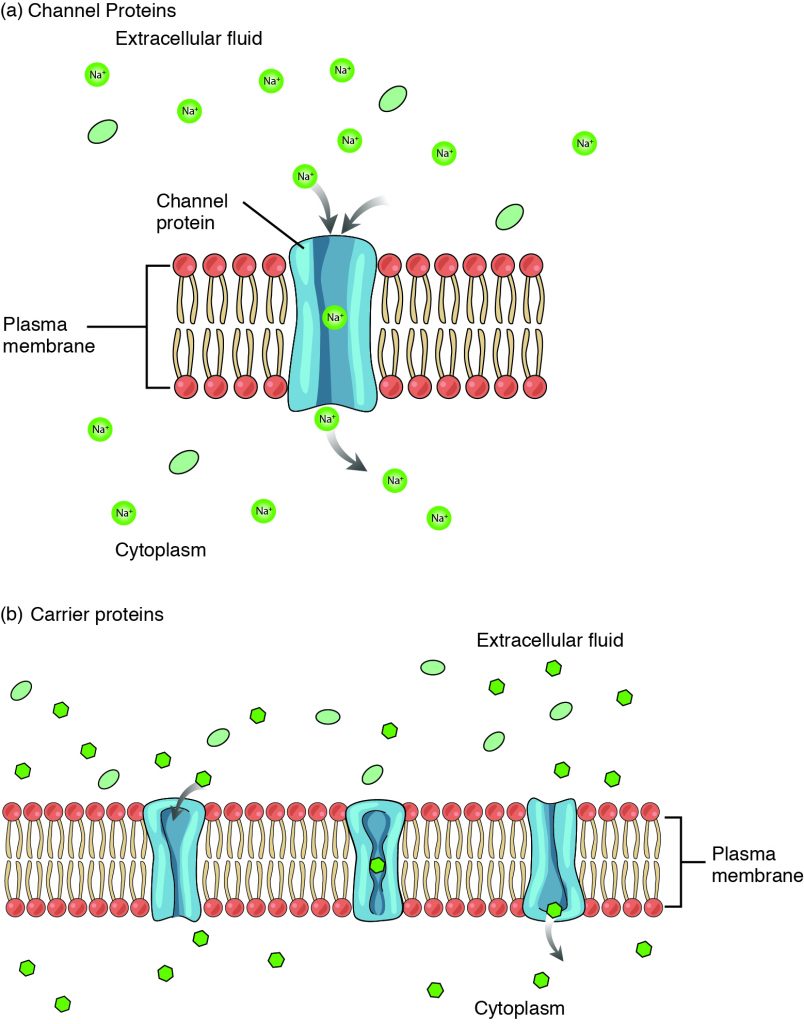
Large polar or ionic molecules, which are hydrophilic, cannot easily cross the phospholipid bilayer. Charged atoms or molecules of any size cannot cross the cell membrane via simple diffusion as the charges are repelled by the hydrophobic tails in the interior of the phospholipid bilayer. Solutes dissolved in water on either side of the cell membrane will tend to diffuse down their concentration gradients, but because most substances cannot pass freely through the lipid bilayer of the cell membrane, their movement is restricted to protein channels and specialized transport mechanisms in the membrane. Facilitated diffusion is the diffusion process used for those substances that cannot cross the lipid bilayer due to their size, charge, and/or polarity, but do so down their concentration gradients (Figure 10.2). As an example, even though sodium ions (Na+) are highly concentrated outside of cells, these electrolytes are charged and cannot pass through the nonpolar lipid bilayer of the membrane. Their diffusion is facilitated by membrane proteins that form sodium channels (or “pores”), so that Na+ ions can move down their concentration gradient from outside the cells to inside the cells. A common example of facilitated diffusion is the movement of glucose into the cell, where it is used to make ATP, using a carrier protein (Figure 10.2b). Although glucose can be more concentrated outside of a cell, it cannot cross the lipid bilayer via simple diffusion because it is both large and polar, and therefore, repelled by the phospholipid membrane. To resolve this, a specialized carrier protein called the glucose transporter will transfer glucose molecules into the cell to facilitate its inward diffusion. The difference between a channel and a carrier is that the carrier usually changes shape during the diffusion process, while the channel does not. There are many other solutes that must undergo facilitated diffusion to move into a cell, such as amino acids, or to move out of a cell, such as wastes.
Osmosis
A specialized example of facilitated transport is water moving across the cell membrane of all cells, through protein channels known as aquaporins. Osmosis is the diffusion of water through a semipermeable membrane from where there is more relative water to where there is less relative water (down its water concentration gradient) (Figure 10.3).
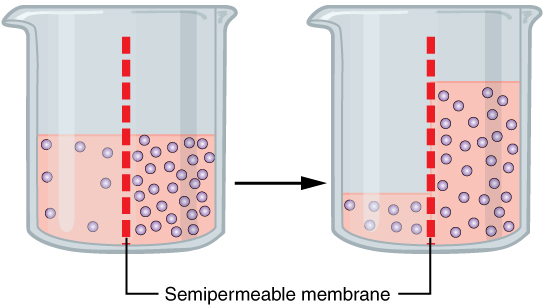
On their own, cells cannot regulate the movement of water molecules across their membrane, so it is important that cells are exposed to an environment in which the concentration of solutes outside of the cells (in the extracellular fluid) is equal to the concentration of solutes inside the cells (in the cytoplasm). Two solutions that have the same concentration of solutes are said to be isotonic (equal tension). When cells and their extracellular environments are isotonic, the concentration of water molecules is the same outside and inside the cells, and the cells maintain their normal shape (and function).
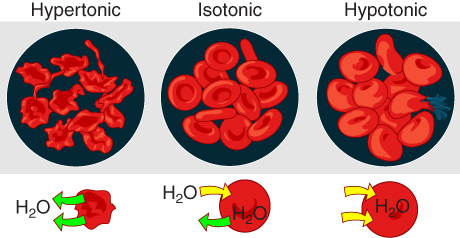
Osmosis occurs when there is an imbalance of solutes outside of a cell versus inside the cell. A solution that has a higher concentration of solutes than another solution is said to be hypertonic, and water molecules tend to diffuse into a hypertonic solution (Figure 10.4). Cells in a hypertonic solution will shrivel as water leaves the cell via osmosis. In contrast, a solution that has a lower concentration of solutes than another solution is said to be hypotonic, and water molecules tend to diffuse out of a hypotonic solution. Cells in a hypotonic solution will take on too much water and swell, with the risk of eventually bursting. A critical aspect of homeostasis in living things is to create an internal environment in which all of the body’s cells are in an isotonic solution. Various organ systems, particularly the kidneys, work to maintain this homeostasis.
Active Transport
For all of the transport methods described above, the cell expends no energy. Membrane proteins that aid in the passive transport of substances do so without the use of ATP. During primary active transport, ATP is required to move a substance against its concentration gradient and across a membrane, with the help of a membrane protein.
One of the most common types of active transport involves proteins that serve as pumps. The word “pump” probably conjures up thoughts of using energy to pump up the tire of a bicycle or a basketball. Similarly, energy from ATP is required for these membrane proteins to transport substances—molecules or ions—across the membrane, against their concentration gradients (from an area of low concentration to an area of high concentration).
The sodium-potassium pump (Figure 10.5), which is also called Na+/K+ ATPase, transports sodium out of a cell while moving potassium into the cell. The Na+/K+ pump is an important ion pump found in the membranes of all cells. The activity of these pumps in nerve cells is so great that it accounts for the majority of their ATP usage.
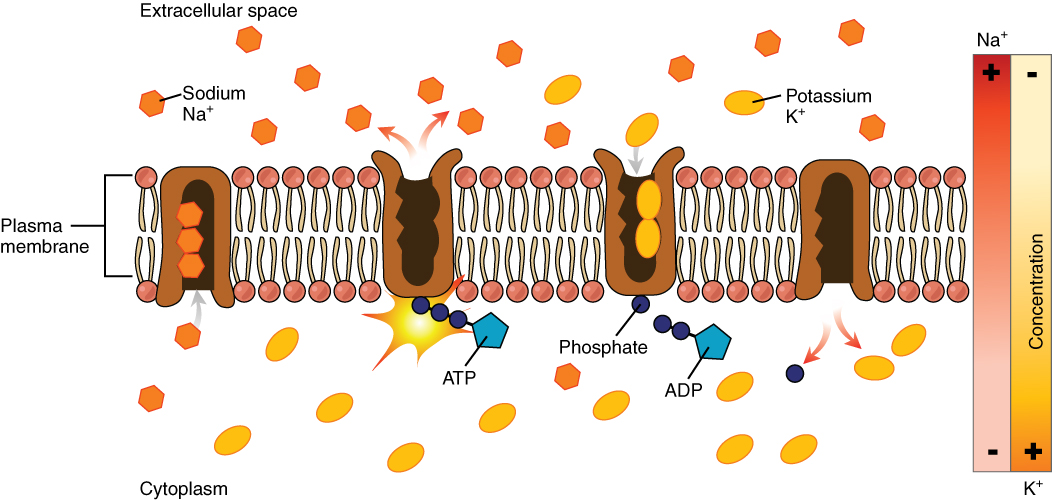
Active transport pumps can also work together with other active or passive transport systems to move substances across the membrane. For example, the sodium-potassium pump maintains a high concentration of sodium ions outside of the cell. Therefore, if the cell needs sodium ions, all it has to do is open a passive sodium channel, as the concentration gradient of the sodium ions will drive them to diffuse into the cell. In this way, the action of an active transport pump (the sodium-potassium pump) powers the passive transport of sodium ions by creating a concentration gradient. When active transport powers the transport of another substance in this way, it is called secondary active transport.
Exocytosis
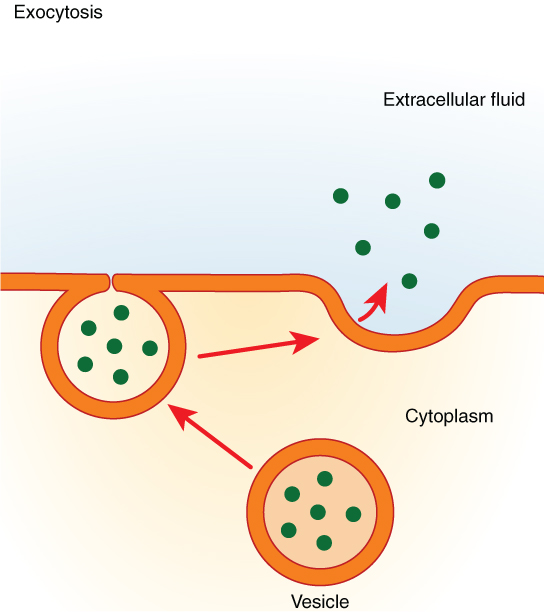
Exocytosis (taking “out of the cell”) is the process of a cell exporting material using vesicular transport (Figure 10.6). Many cells manufacture substances that must be secreted, like a factory manufacturing a product for export. These substances are typically packaged into membrane-bound vesicles within the cell. When the vesicle membrane fuses with the cell membrane, the vesicle releases its contents into the interstitial fluid. The vesicle membrane then becomes part of the cell membrane.
Specific examples of exocytosis include cells of the stomach and pancreas producing and secreting digestive enzymes and endocrine cells producing and secreting hormones that are sent throughout the body.
The addition of a new membrane to the plasma membrane is usually coupled with endocytosis so that the cell is not constantly enlarging. Through these processes, the cell membrane is constantly renewing and changing as needed by the cell.
a substance that repels water
the ability of a membrane to control the movement of substances into and out of the cell
molecules that have electrons that are shared equally throughout; molecules with a consistent charge
movement of substances across the cell membrane without the expenditure of cellular energy
movement of substances across the cell membrane using energy from adenosine triphosphate (ATP)
A difference in the amount of a solute in two solutions
movement of substances from areas of high to low concentration
form of passive transport in which an ion moves directly through the membrane, down its concentration gradient
body fluids located outside of cells
molecules that share electrons unequally, creating differences in charge throughout
a substance that is attracted to water
a form of passive transport in which ions are moved across the membrane with protein interaction
proteins that change in shape to allow substances to pass through the membrane
net movement of water across a semi-permeable from an area where it is higher in volume to an area where it is lower in volume; the movement of water down its concentration gradient
cellular structure that houses fluid and organelles
solutions that have equal osmolarities; no net fluid of movement
solution in which the fluid osmolarity is greater than the cell osmolarity; net fluid movement out of the cell
solution in which the fluid osmolarity is less than the cell osmolarity; net fluid movement into the cell
cellular active transport pump responsible for transporting sodium out of a cell and moving potassium into the cell
the discharge of substances out of the cell and into the extracellular space by the transport of secretory vesicles
a sub-compartment of extracellular fluid that bathes cells and provides a medium of exchange for substances between the blood and cells

